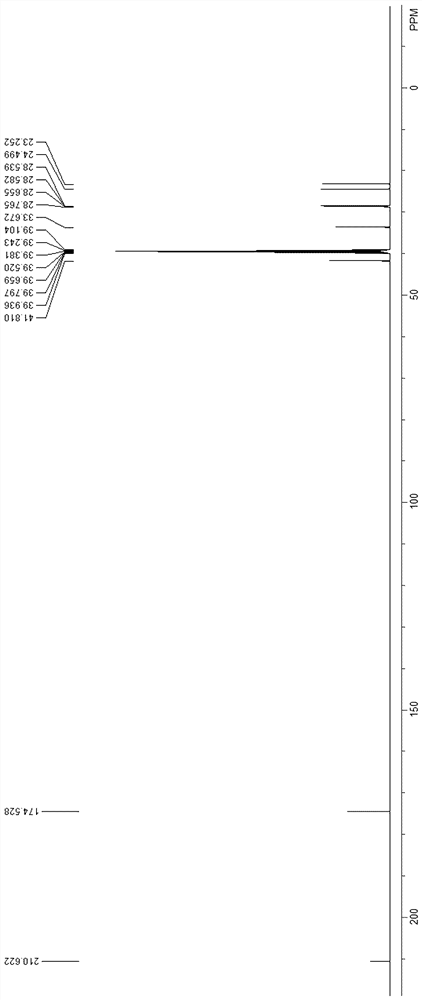
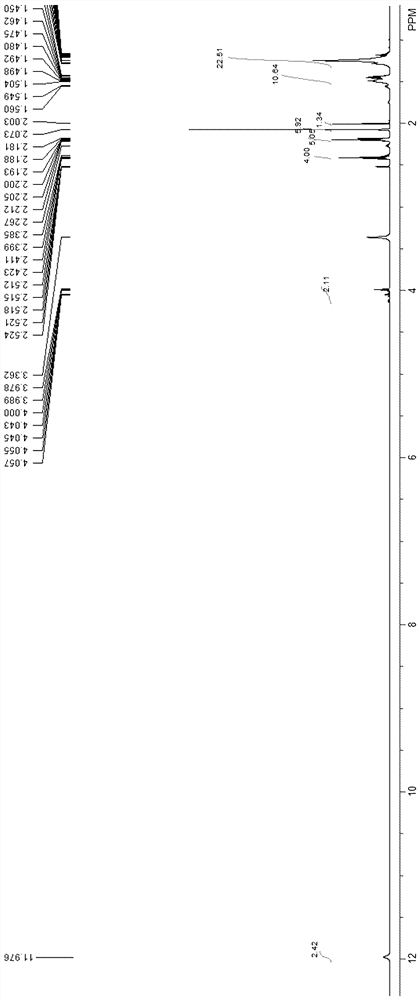
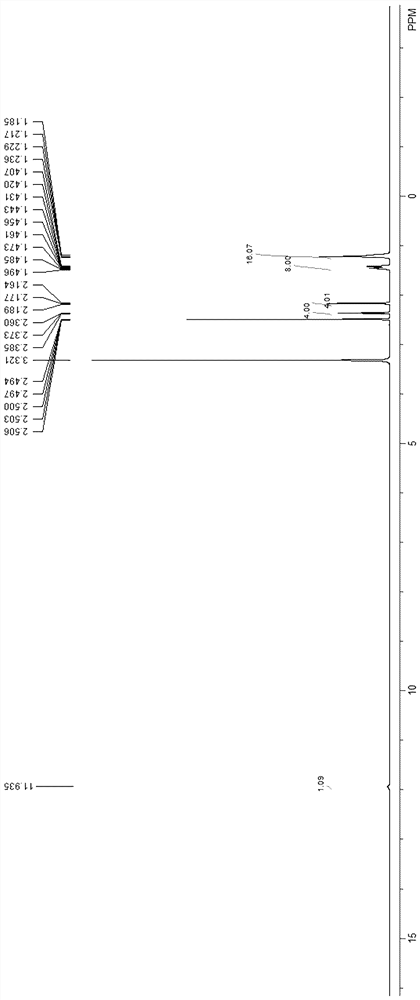
Preparation method of 10-oxononadecanedioic acid
A technology of oxononadecane and diacid, which is applied in the field of preparation of 10-oxononadecanedioic acid, can solve the problems of difficult control of the reaction process, harsh reaction conditions and high equipment requirements, and achieves easy operation and control, The effect of mild reaction conditions and great economic potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Dimethyl sebacate (compound (III)) (1.0 equiv, 170 g, 738.2 mmol) was weighed in a 2000 mL round-bottom flask, dissolved in N,N-dimethylformamide (850 mL, 5 times the volume), cooled To -10°C, potassium hydroxide powder (1.1 equiv) was added and the reaction was allowed to proceed overnight at -10°C, the solution gradually turned from clear to white cloudy. After the reaction is completed, add 2 L of water to dilute, extract three times with 500 mL of ethyl acetate, and recover the unconverted raw material dimethyl sebacate. The aqueous phase was adjusted to pH=3 with dilute hydrochloric acid, extracted three times with 500 mL of ethyl acetate, and the solvent was removed by rotary evaporation to obtain a crude product, which was purified by silica gel column chromatography (ethyl acetate:n-hexane=1:5, V / V) to obtain Monomethyl sebacate (compound (IV)) 121 g, yield 76%, white solid, purity detected by HPLC normalization method was 99%.
[0095] LC-MS (ESI): Calculated ...
Embodiment 2
[0099]In a 500 mL round-bottomed flask were weighed monomethyl sebacate (compound (IV)) (1.0 equiv, 5.1 g, 23.57 mmol), dimethylamine hydrochloride (1.3 equiv, 2.5 g, 30.64 mmol), EDCI (1.5 equiv. , 6.78 g, 35.36 mmol), DMAP (0.1 equiv, 288 mg, 2.36 mmol), dissolved in 50 mL of dichloromethane, finally added pyridine (2.8 equiv, 5.22 g, 66 mmol), and stirred at room temperature overnight. After the reaction, 50 mL of water was added to dilute, adjusted to pH=3 with dilute hydrochloric acid, the organic phase was separated, the aqueous phase was extracted twice with 70 mL of dichloromethane, the organic phases were combined, washed with water, washed with saturated sodium chloride solution, and washed with anhydrous sulfuric acid. It was dried over sodium and concentrated to obtain 4.8 g of methyl 10-(dimethylamino)-10-oxodecanoate (compound (V)), with a yield of 84%, as a brownish yellow oil, which was directly used in the next step without further purification. step.
[0100...
Embodiment 3
[0104] In a 250mL three-necked flask equipped with two 100mL constant pressure dropping funnels and three-way nitrogen balloons, weigh 10-(dimethylamino)-10-oxodecanoic acid methyl ester (compound (V)) (1.0 equiv., 10 g, 41.09 mmol), dissolved in 50 mL of dichloromethane, replaced with nitrogen protection, cooled the device to 0 °C, weighed titanium tetrachloride (1.2 equiv, 9.35 g, 49.31 mmol) and injected it into a constant pressure dropping funnel with a syringe It was added dropwise to the reaction solution, and the solution was kept for 5 minutes after the dropwise addition was completed. At this time, the solution was orange. Then, triethylamine (1.4 equivalents, 5.82 g, 57.53 mmol) was weighed and injected into a constant pressure dropping funnel with a syringe for dropwise addition. In the reaction solution, the reaction solution turned black and white smoke was generated. After the dropwise addition was completed, the reaction was kept at a temperature for 1 hour. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com