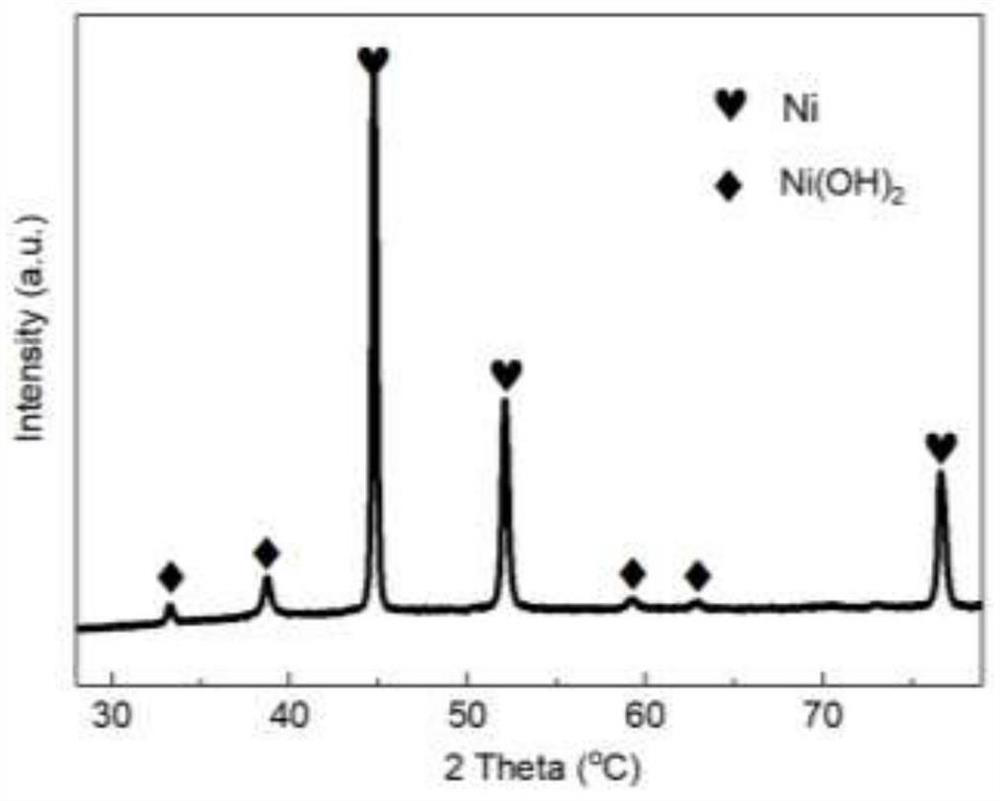
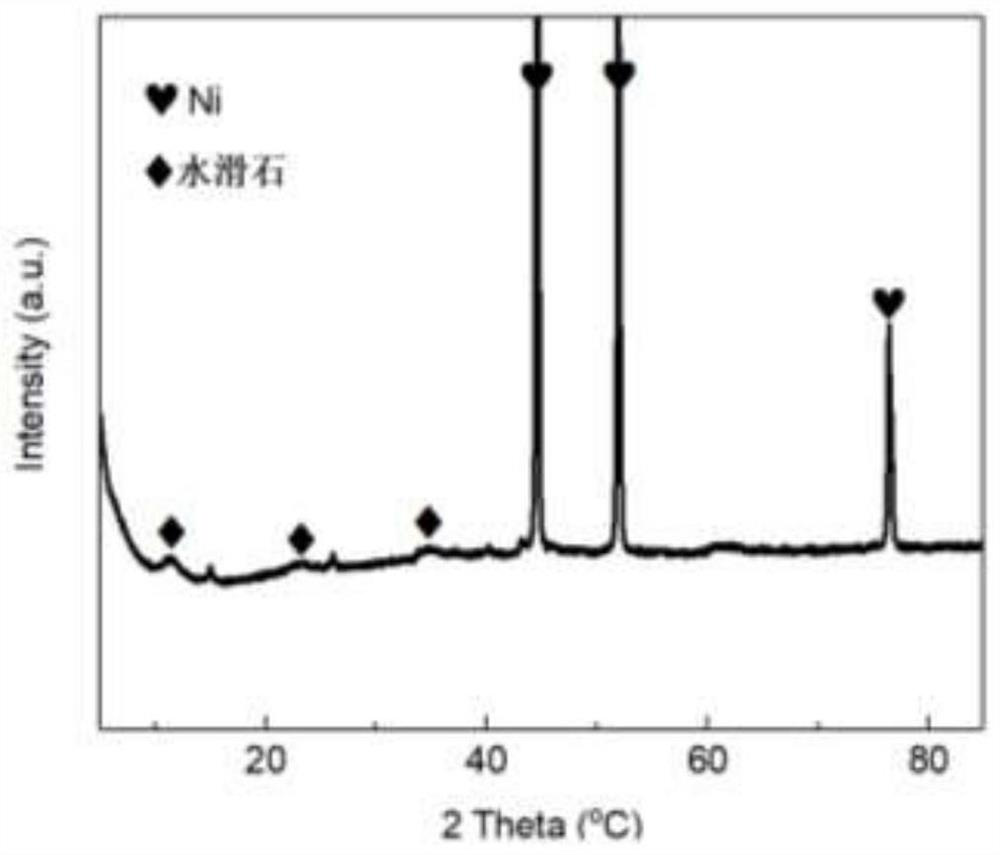
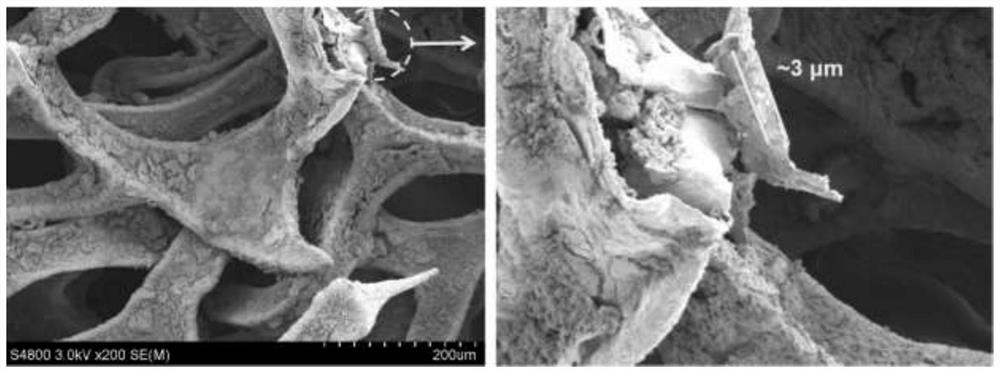
Nickel-based catalyst derived from self-supporting layered double hydroxides as well as preparation method and application of nickel-based catalyst
A layered bimetallic, nickel-based catalyst technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, organic compound/hydride/coordination complex catalyst, hydrogenation preparation, etc., can solve the problem of low reaction conversion rate and other problems, to achieve the effect of high reaction selectivity and improved conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] This example provides a self-supporting layered double metal hydroxide derived nickel-based catalyst.
[0084] 1) Weigh 10g nickel foam, 50mL sodium carbonate solution, and ultrasonically treat for 30 minutes to remove surface stains; after washing with distilled water, ultrasonically treat with dilute hydrochloric acid for 30 minutes to remove surface oxide film; after washing with distilled water, place in 100mL hydrothermal reactor, add 60mL Aqueous ammonium chloride solution (1 mol / L) was reacted at 180° C. for 18 hours; the reaction was completed, washed with distilled water and dried to obtain a nickel hydroxide crystal layer grown in situ on the foamed nickel substrate.
[0085] 2) Prepare an aqueous solution of aluminum nitrate (0.3mol / L), an aqueous solution of sodium citrate (0.2mol / L), and an aqueous solution of sodium hydroxide, and respectively introduce nitrogen gas to remove carbon dioxide gas in the aqueous solution.
[0086] 3) In a nitrogen atmosphere,...
Embodiment 2
[0095] This embodiment provides a nickel-based catalyst.
[0096] 1) Weigh 10g of nickel fibers, 50mL of sodium carbonate solution, and ultrasonically treat for 30 minutes to remove surface stains; after washing with distilled water, ultrasonically treat with dilute hydrochloric acid for 30 minutes to remove surface oxide film; after washing with distilled water, place in a 100mL hydrothermal reactor, add 60mL Aqueous urea solution (5 mol / L) was reacted at 150° C. for 12 hours; the reaction was completed, washed with distilled water and dried to obtain a nickel hydroxide crystal layer grown in situ on the nickel fiber matrix.
[0097] 2) Configure an aqueous solution of aluminum nitrate (0.3mol / L), an aqueous solution of cobalt nitrate (0.05mol / L), an aqueous solution of sodium citrate (0.2mol / L), and an aqueous solution of sodium hydroxide, and respectively introduce nitrogen to remove carbon dioxide gas in the aqueous solution.
[0098] 3) In a nitrogen atmosphere, the nicke...
Embodiment 3
[0102] 1) with step 1 in Example 2);
[0103] 2) configure aluminum nitrate aqueous solution (0.3mol / L), sodium citrate aqueous solution (0.2mol / L), sodium hydroxide aqueous solution, respectively feed nitrogen to remove carbon dioxide gas in the aqueous solution;
[0104] 3) In a nitrogen atmosphere, the nickel fibers with the nickel hydroxide crystal layer grown in situ were added to a 250mL three-necked flask, the three-necked flask was placed in an ultrasonic water bath, the temperature of the water bath was controlled at 30°C, and the ultrasonic power was 300W; 30mL was added to remove After the aluminum nitrate (0.3 mol / L) aqueous solution of carbon dioxide, 30 mL of sodium citrate aqueous solution (0.2 mol / L) to remove carbon dioxide was added dropwise; after the dropwise addition, the temperature of the water bath was raised to 60 ° C, and then the hydrogen that removed carbon dioxide was added dropwise. The sodium oxide aqueous solution was adjusted to pH 10 and aged ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com