Method for improving bacitracin produced by bacillus licheniformis
A technology for Bacillus licheniformis and bacitracin, which is applied in the field of improving the production of bacitracin by Bacillus licheniformis, can solve the problems of reduced yield of target products, loss of carbon atoms, etc., and achieves the effects of reducing growth toxicity, being easy to operate, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Screening of Bacillus licheniformis ATH strains
[0061] (1) After incubating the feed samples in a 28°C incubator for 24 hours, the feed samples were treated in a water bath at 80-85°C for 15 minutes, cooled to room temperature and then diluted with physiological saline. The sample dilution was spread on the bacitracin screening medium plate, and after culturing at a constant temperature of 37°C for 24 hours, the colonies that grew and full on the medium were picked and cultured in LB liquid medium.
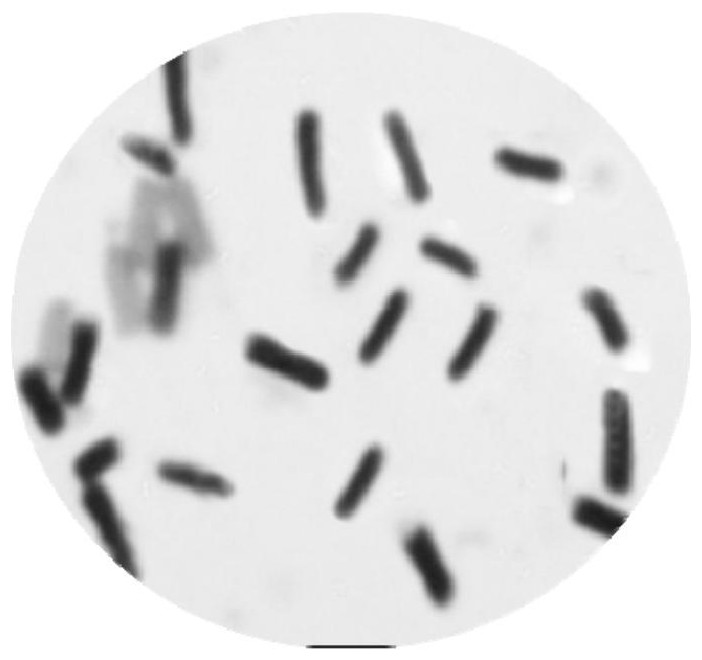
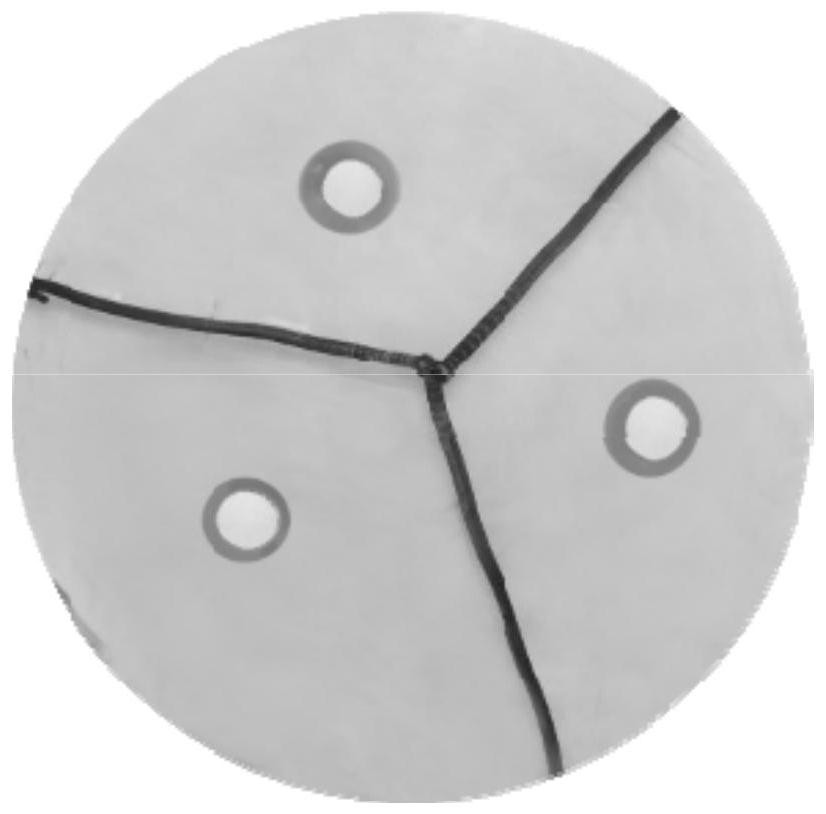
[0062] (2) The single colony after screening is such as figure 1 As shown, the colonies are round, with uneven edges, thin, rough surface, opaque, and grayish white. The results of single-stain microscopy are as follows: figure 2 It was shown that the bacterial cells were about 1.5-3.0 μm in length and 0.6-0.8 μm in width, and the strains were Gram-positive. The 16S rDNA (nucleotide sequence shown in SEQ ID NO. 3) of the screened strain was sequenced, and th...
Embodiment 2
[0063] Example 2: Bacteriostatic test of Bacillus licheniformis ATH
[0064] (1) Screening of indicator strains
[0065] In order to verify the bacteriostatic effect of Bacillus licheniformis ATH, Escherichia coli, Bacillus cereus, Corynebacterium glutamicum, and Staphylococcus aureus were selected as indicator strains in this experiment;
[0066] After culturing the above indicated strains at 37°C for 12 hours overnight, diluting OD=0.8 with sterile water on an ultra-clean workbench, taking 200 μL and spreading them on LB plates as bacteriostatic indicator plates.
[0067] (2) Bacillus licheniformis ATH was inoculated into LB liquid medium, cultured at 37°C overnight, the bacterial concentration of Bacillus licheniformis ATH was diluted to OD600=1.0, and then filtered with a 0.22 μm microporous membrane in an ultra-clean workbench. sterilized filter paper with a diameter of 2 cm to absorb the quantitative bacterial solution, respectively point and inoculate it on the antibac...
Embodiment 3
[0082] Example 3: Construction of Acetyl-CoA synthase expression vector
[0083] Specific steps are as follows:
[0084] (1) The genomic DNA of Bacillus subtilis subsp 168 was used as a template, and F and R were used as primers for PCR amplification to obtain the heterologous acetyl-CoA synthase gene Bsacs (the nucleotide sequence is shown in SEQ ID NO.1). ); the primer sequences used are as follows:
[0085] F: 5-ATGGGTCGCGGATCCGATGAACTTGAAAGCGTTAC-3';
[0086] R: 5-CGAGTGCGGCCGCAATTAATCCTCCATTGTTGACAG-3'.
[0087] PCR amplification conditions were: 95°C pre-denaturation, 3 min; 95°C denaturation, 30s, 58°C annealing, 30s, 72°C extension, 90s, 30 cycles; 72°C final extension for 10 min.
[0088] The PCR amplification system was: template 0.5 μL, upstream and downstream primers 0.5 μL each, sterilized double-distilled water 23.5 μL, PrimerStar DNA polymerase 25 μL.
[0089] (2) ligating the gene Bsacs (with pHY300PLK plasmid) encoding acetyl-CoA synthase obtained in step ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com