Anode material of lithium ion cell and preparation method
A technology for lithium-ion batteries and positive electrode materials, applied in the field of lithium-ion battery positive electrode materials and their preparation, can solve the problems of reducing the cycle performance of positive electrode materials, decreasing the thermal stability and safety of batteries, and generating oxygen, etc., and achieving good high-rate discharge Performance, good anti-overcharge performance, good high temperature performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
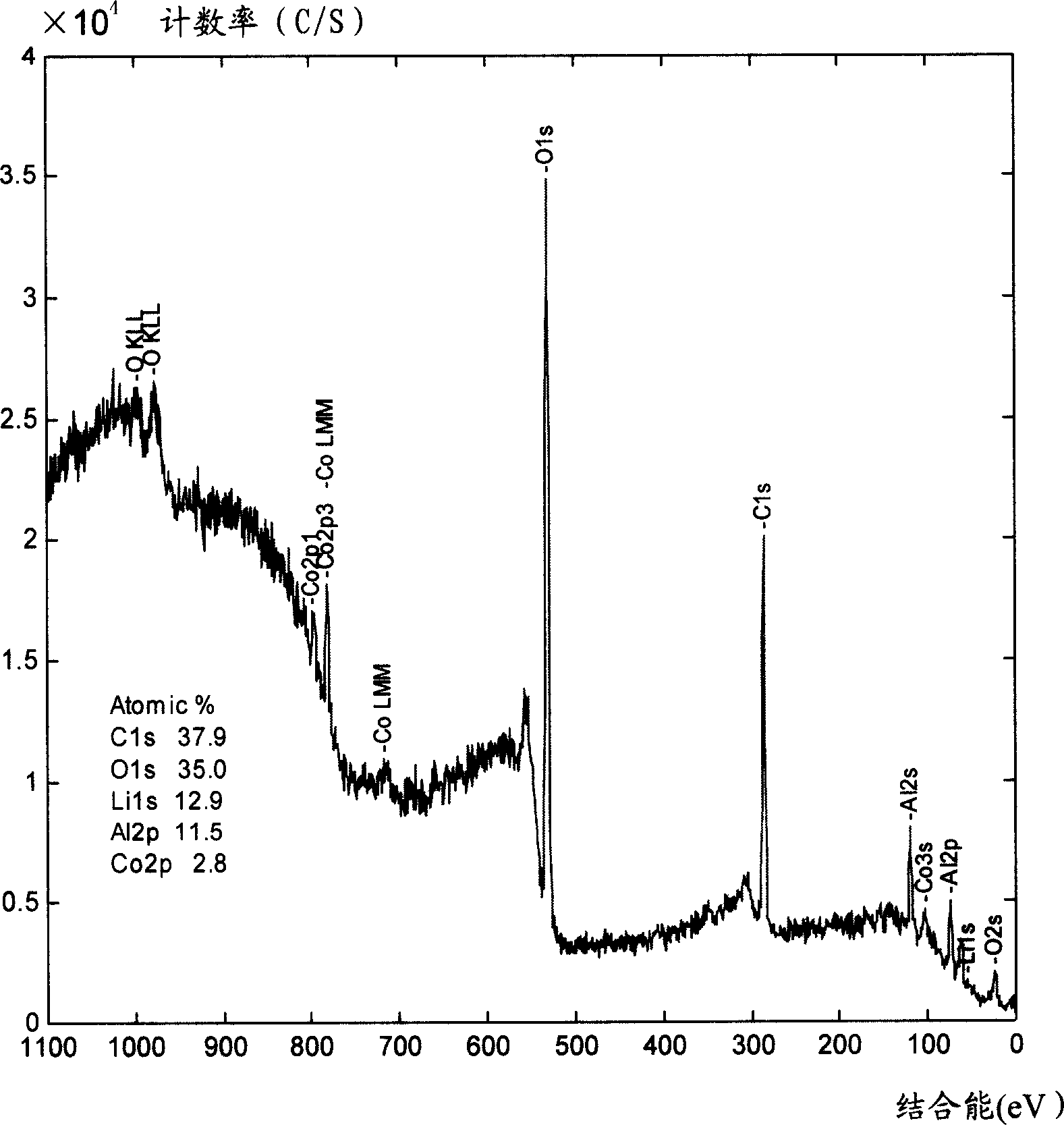
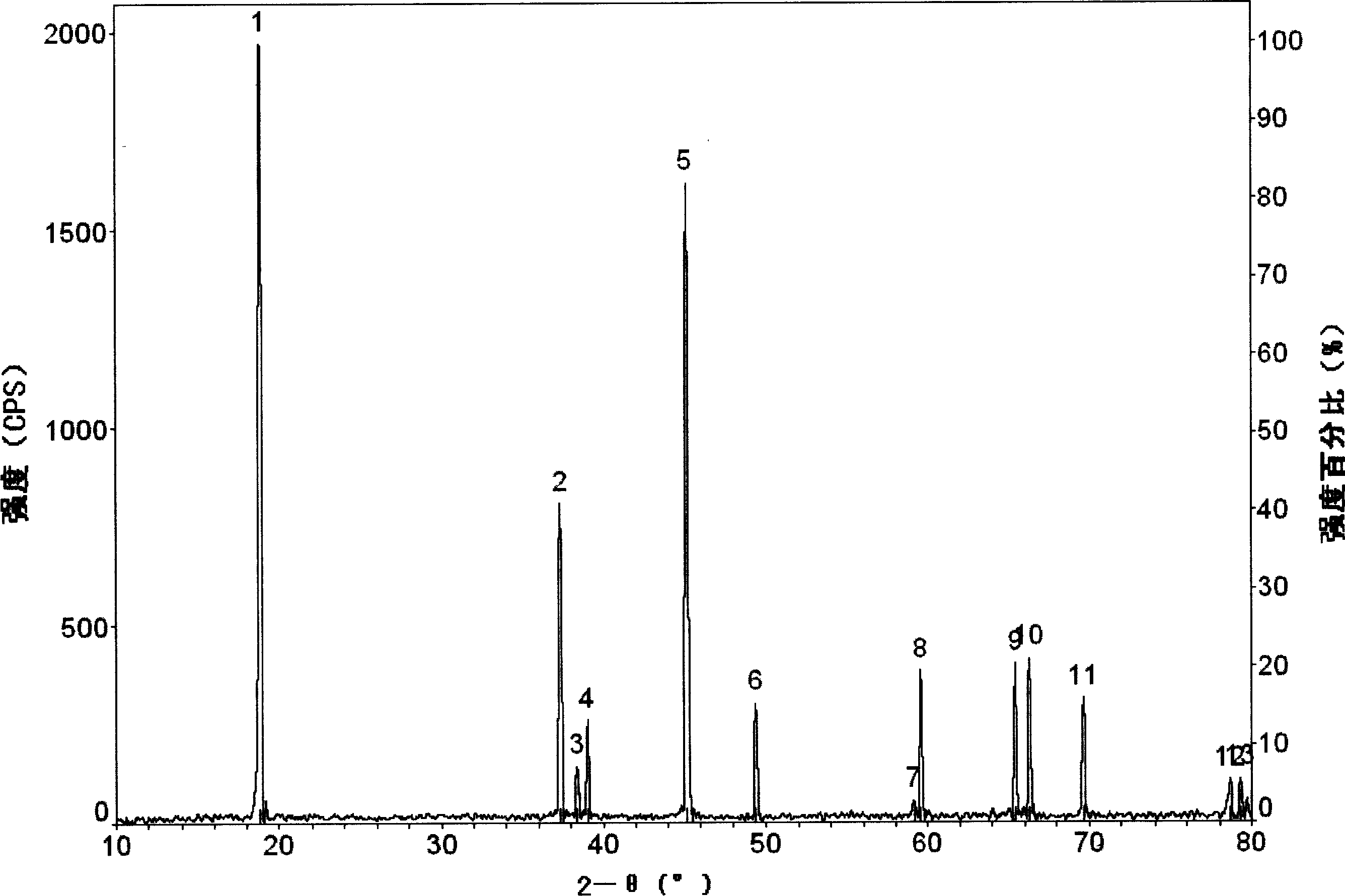
[0030] The preparation method of the cathode material of the lithium ion battery of the present invention is prepared by a liquid phase method. After the additive is heat-treated, an oxide coating layer is formed on the surface of the positive electrode active material, so that the structural change of the positive electrode active material during charging and discharging is relatively small. The positive active material can be lithium cobaltate LiCoO 2 , lithium nickel cobalt oxide LiNi 1-X co X o 2 (01 / 3 co 1 / 3 mn 1 / 3 o 2 , lithium manganese oxide LiMn 2 o 4 At least one of them has an average particle size of 4-12 microns, preferably 10-12 microns, because the larger the particle size, the more obvious the effect of the additive. And if the particle size of the positive electrode active material is too small, it is easy to form a blend with the additive instead of forming a uniform coating layer on the surface of the positive electrode active material, and the oxide...
Embodiment 1
[0036] Preparation of positive electrode material: Li 2 CO 3 and Co 3 o 4 Mix evenly, and heat-treat at 900°C for 10 hours to obtain lithium cobaltate with an average particle size of 10-12 microns. Dissolve aluminum nitrate whose aluminum weight accounts for 0.1% by weight of lithium cobaltate in absolute ethanol, add lithium cobaltate, mix well, evaporate the solvent, and then heat treat at 800° C. for 4 hours to obtain the positive electrode material of the present invention.
[0037] Preparation of the positive electrode sheet: 85% by weight of the resulting positive electrode material, 10% by weight of graphite as a conductive agent and 5% by weight of PVDF (polyvinylidene fluoride) as a binding agent, joined together in NMP (methyl-2-pyrrolidinone) solvent, It is fully stirred into a paste to obtain a positive electrode material slurry mixture. This slurry is uniformly coated on both sides of strip-shaped aluminum foil, and after drying, it is pressed into a strip-sh...
Embodiment 2
[0042] Except that the addition of aluminum element in aluminum nitrate increases to 5% by weight of lithium cobaltate, the rest are the same as
[0043] Example 1 is the same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com