Method for constructing genetic engineering fungus of monascus with no citrinin
A technology of genetically engineered bacteria and a construction method, which is applied in the field of constructing and breeding high-yield engineered bacteria that do not produce citrinin and Monascus pigment, can solve problems such as pollution and concomitant occurrence, and achieve safety assurance, quality improvement, and solution to citrinin Effects of toxin concomitant and pollution problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] Embodiment 1: the cloning of CICC 5006 citrinin coding gene
[0226] Primer design: Two pairs of primers were designed based on the gene sequence of M. purpureus citrinin released by GeneBank, pksCT-F: 5′-GGCCGCGGCCGCGCTTCTTACCAACTTCCCTT-3′, pksCT-R: 5′-GGCCGTTAACACCTTCAGTCCGCTATCTATC-3′.
[0227] CICC5006 Chromosomal DNA Extraction Method
[0228] Take 1g of CICC5006 fresh mycelium and place it in a pre-cooled mortar, add appropriate amount of quartz sand, add 1mL CTAB extraction buffer, add proteinase K to a final concentration of 0.1mg / mL, grind on ice to form a uniform turbid solution, transfer to 5mL Centrifuge tube. Add 1 / 10 volume of 10% SDS, bathe in 65°C water bath for 25min-30min, then cool to room temperature. Extract twice with an equal volume of mixed solvent phenol: chloroform: isoamyl alcohol (25:24:1), transfer the upper aqueous phase to a new centrifuge tube, add RNase I (RNase I) without DNase (DNase) activity To a final concentration of 20ug / mL, in...
Embodiment 2
[0233] Embodiment 2: Construction of knockout vector
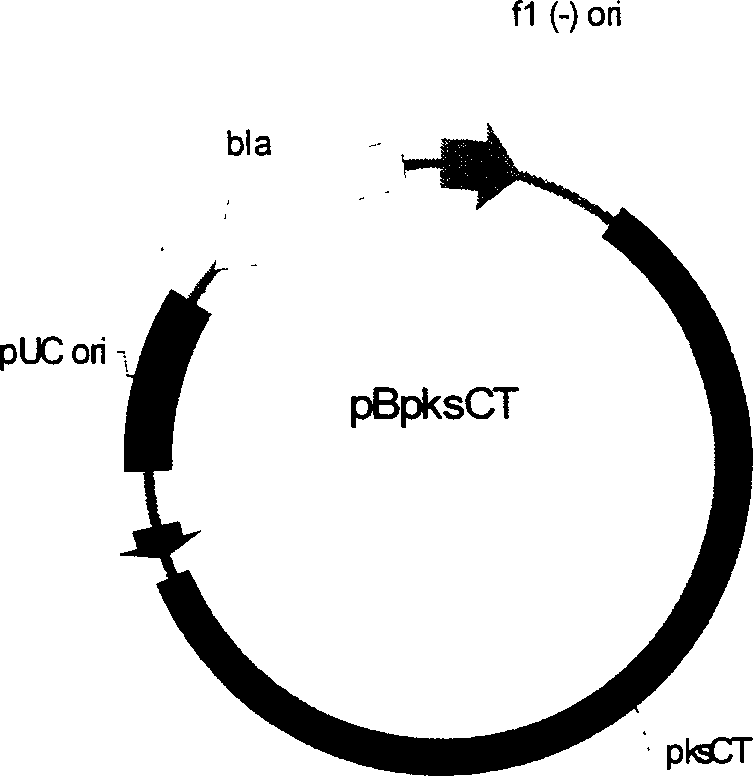
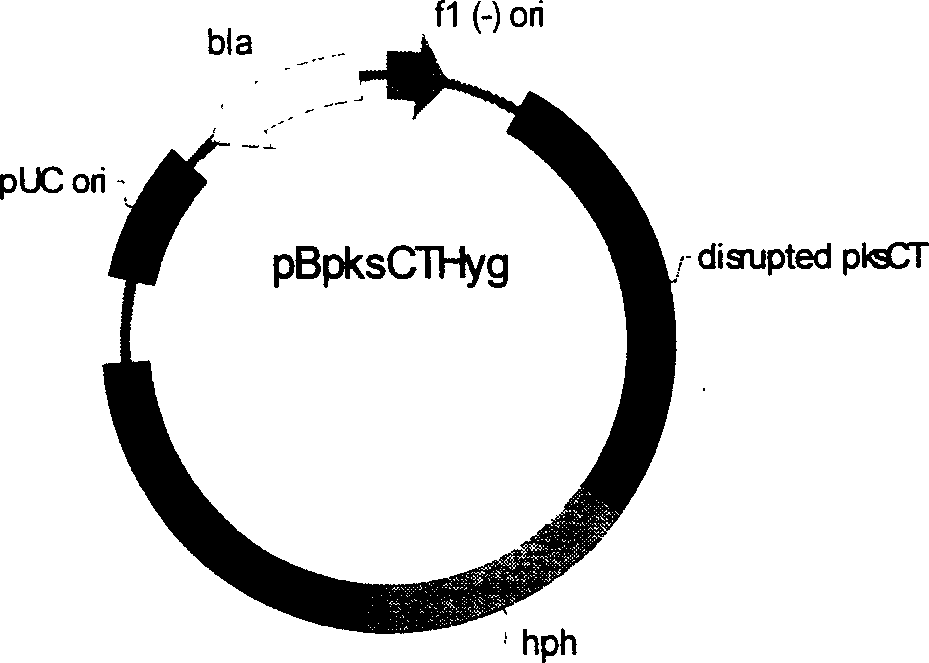
[0234] The Hpa I restriction fragment from the vector pBC1003 1.4kb (this fragment contains the promoter from the Aspergillusnidulans tupC gene and the hygromycin B resistance gene hph sequence) is inserted into the Sma I site of the vector pBpksCT (the Sma I site is located in the pksCT gene sequence 2253bp inside) was constructed to obtain the monascus citrinin knockout vector pBpksCTHyg. The vector pBpksCTHyg knocked out the CICC5006 citrinin gene through REMI (restrictedenzyme-mediated integration) to construct a citrinin-free high-yield engineered strain.
Embodiment 3
[0235] Embodiment 3: REMI-mediated gene knockout method
[0236] Preparation of protoplasts
[0237] Strain CICC5006 was cultured on spore-forming medium at 30°C for 7d to 10d, and the spores were collected and prepared into a uniform spore suspension (1×10 8 pieces / ml). Take 200ul potato agarose solid plate coated with cellophane, incubate at 30°C for 30h, collect mycelium, 1mol / L MgSO 4 Solution washed once. Add 30ml of lysing enzyme solution (0.3% lysing enzyme, 0.1% cellulase and 1% snailase) to the harvested mycelia on each plate, and hydrolyze at 30°C and 60r / min. Filter the protoplast body fluid, centrifuge the filtrate at 3200r / min for 10min, discard the supernatant, resuspend the precipitate in a pre-cooled 1.0mol / L sorbitol solution, and centrifuge. The protoplast pellet was resuspended in 1.0mol / L sorbitol, and placed in an ice bath for later use.
[0238] REMI-mediated gene knockout method
[0239] Method 1. The above-mentioned protoplast body fluid is centri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com