Reagent and method for separating and determining dissociative DNA in blood
A technology of reagents and kits, which is applied in the field of medical oncology, can solve the problems of unsuitability for quantitative analysis of free blood DNA and large errors, and achieve the effects of low cost, high DNA yield, and short DNA separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the establishment of real-time quantitative PCR detection method
[0037] 1.1 Materials and methods
[0038] A. Materials: Human A431 epithelioid cancer cell line was purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. Fetal bovine serum and F12 medium were purchased from GIBCO. Tri Reagent RNA extraction kit was purchased from Molecular Research Center, USA. Revert Aid TM The first-strand cDNA synthesis kit was purchased from MBI Fermantas, Lithuania. PCR primers and fluorescent probes were synthesized by Shanghai Saibaisheng Gene Technology Co., Ltd. The sequencing of PCR products was completed by Treasure Bioengineering (Dalian) Company. Ex Taq DNA polymerase and pMD 18-T PCR product cloning vector and real-time quantitative PCR kit were purchased from Bao Biological Engineering (Dalian) Co., Ltd.
[0039] B. Design of primers and probes: According to the Genbank database, the human epithelial growth fa...
Embodiment 2
[0049] Example 2: Linear relationship of genomic DNA detection
[0050] 2.1 Materials and methods
[0051] 2 cases of normal lung tissue were surgical resection specimens of lung cancer. The lung tissue above 5cm away from the cancer tissue was selected and stored in liquid nitrogen. DNAezsol Genomic DNA Extraction Kit was purchased from Shanghai Shenergy Bocai Biotechnology Company.
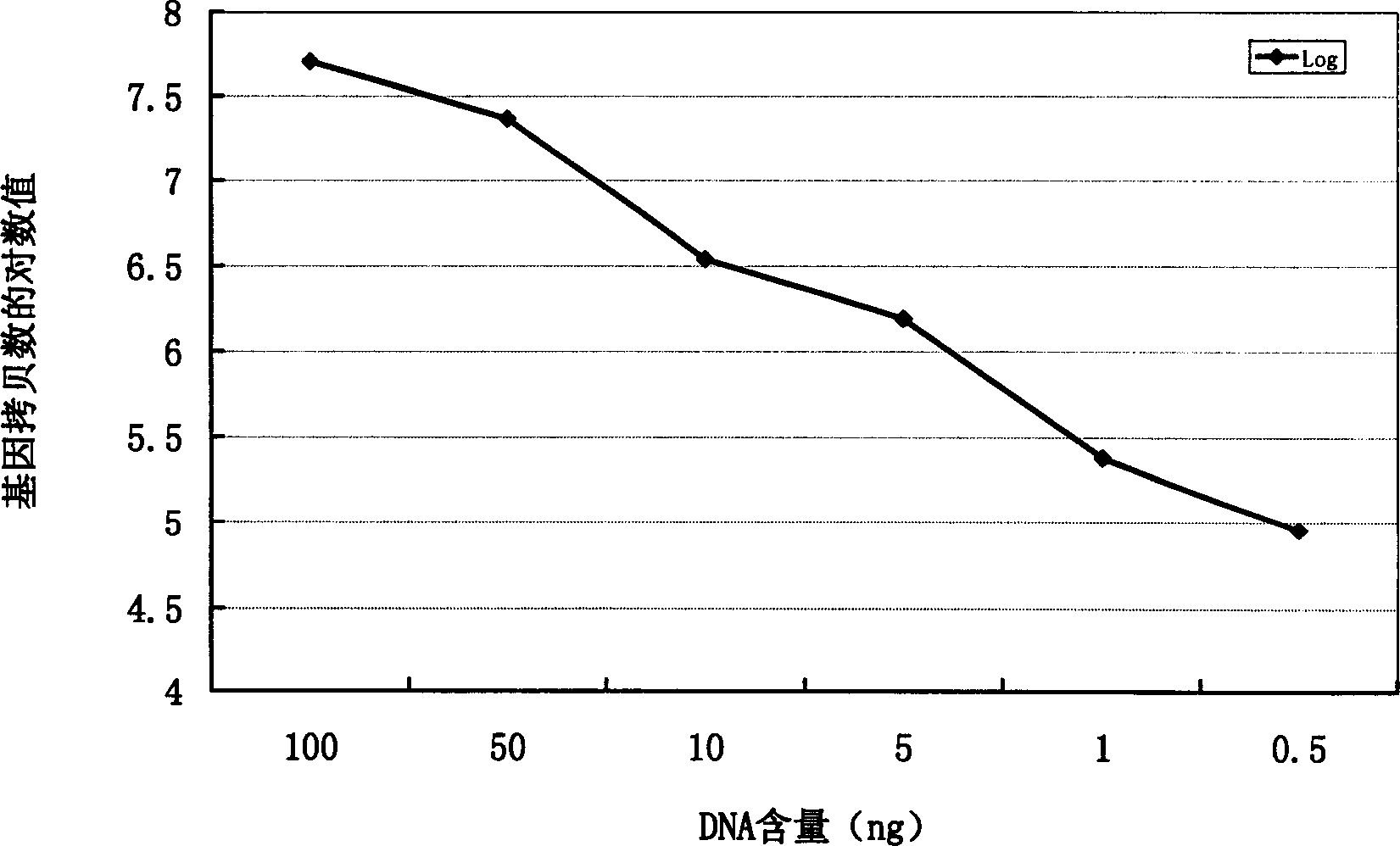
[0052] Take 50 mg of lung tissue and extract DNA according to the operating instructions. The obtained genomic DNA is measured by ultraviolet spectrophotometry for A260 and A280, requiring A260 / A280>1.80. Then sample DNA was taken for serial dilution, the concentrations were 50, 25, 5, 2.5, 0.5 and 0.25 ng / μl, and 2 μl of each was taken for quantitative PCR detection.
[0053] 2.2 Results
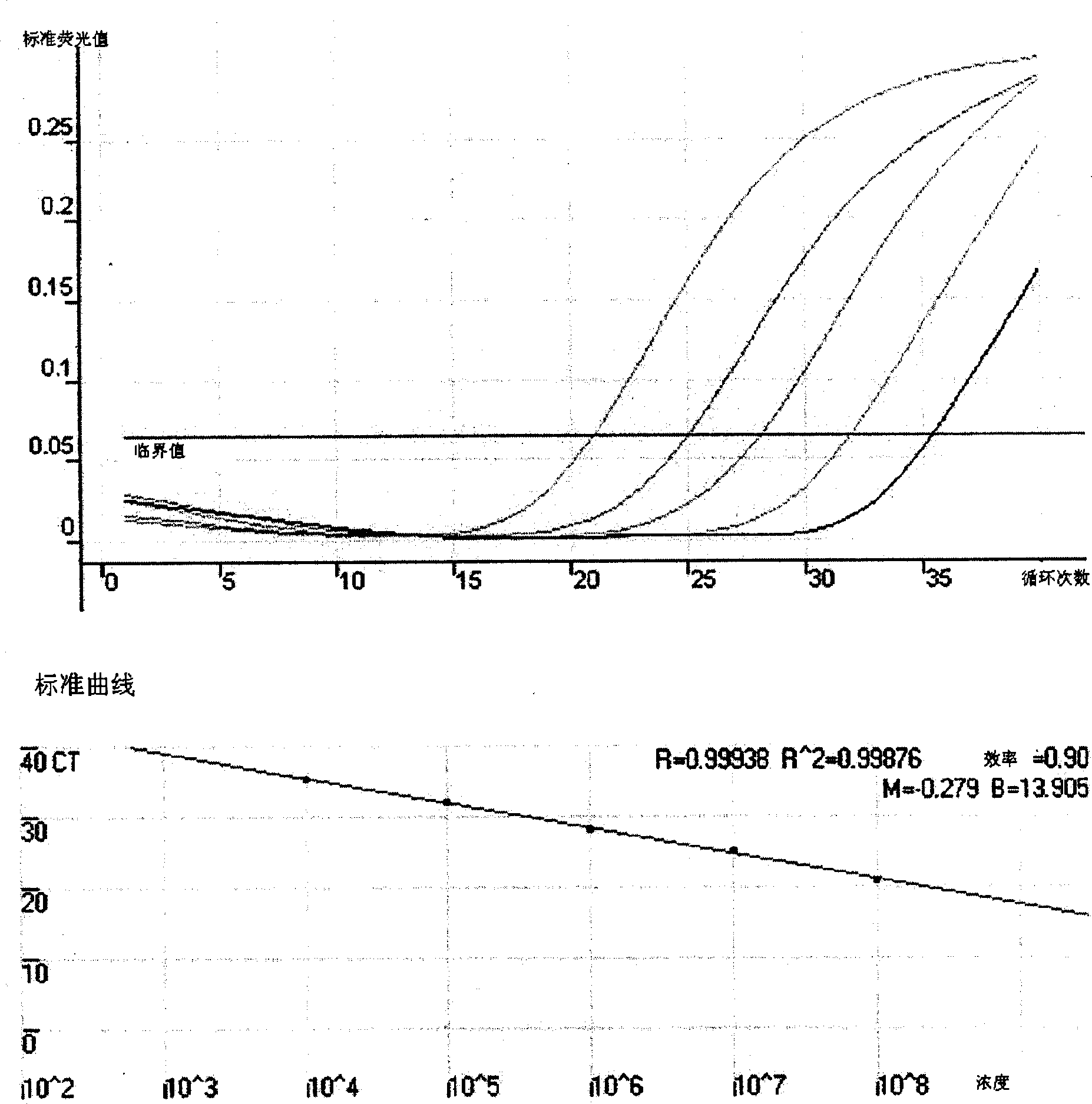
[0054] image 3 The results showed that the real-time quantitative detection of genomic DNA in normal lung tissue had a detection limit of at least 0.5 ng / reaction, and there was a linear relationship betw...
Embodiment 3
[0055] Embodiment 3: Quantitative analysis of serum free DNA
[0056] 3.1 Materials and methods
[0057] There were 10 serum samples from healthy people and 42 serum samples from lung cancer patients. All patients were confirmed by cytology or histology. The serum preparation method is as follows: 3ml of blood is extracted intravenously, placed in a clean centrifuge tube overnight at 4°C, then centrifuged at 1500 rpm for 5 minutes, and the upper layer of serum is kept in a -70°C refrigerator.
[0058] The extraction of serum free DNA adopts the blood free DNA extraction reagent a) that above-mentioned embodiment 3 makes, and its operating steps are as follows:
[0059] 1. Add 500 microliters of blood cell-free DNA extraction reagent a) into 1.5ml Eppendorf tube, and preheat at 95°C for 5 minutes;
[0060] 2. Add 100-500 microliters of plasma, heat at 95°C for 5 minutes, and shake vigorously for 20 seconds;
[0061] 3. Centrifuge at 13,000rpm for 5min;
[0062] 4. Transfer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gene copy number | aaaaa | aaaaa |
| Gene copy number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com