Method for treating fibrosis
a fibrosis and fibrosis technology, applied in the field of fibrosis treatment, can solve problems such as tissue damage and physical injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
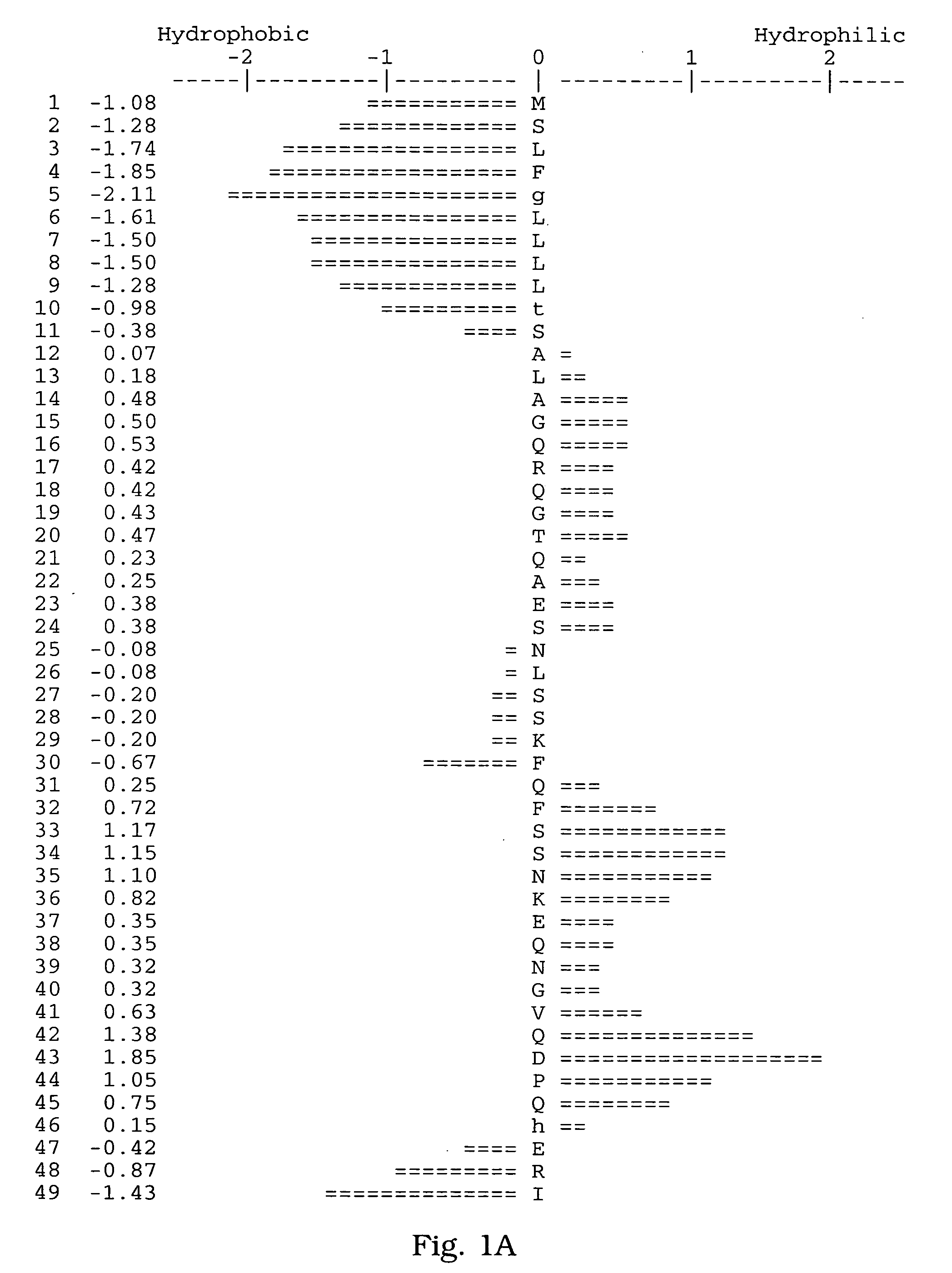
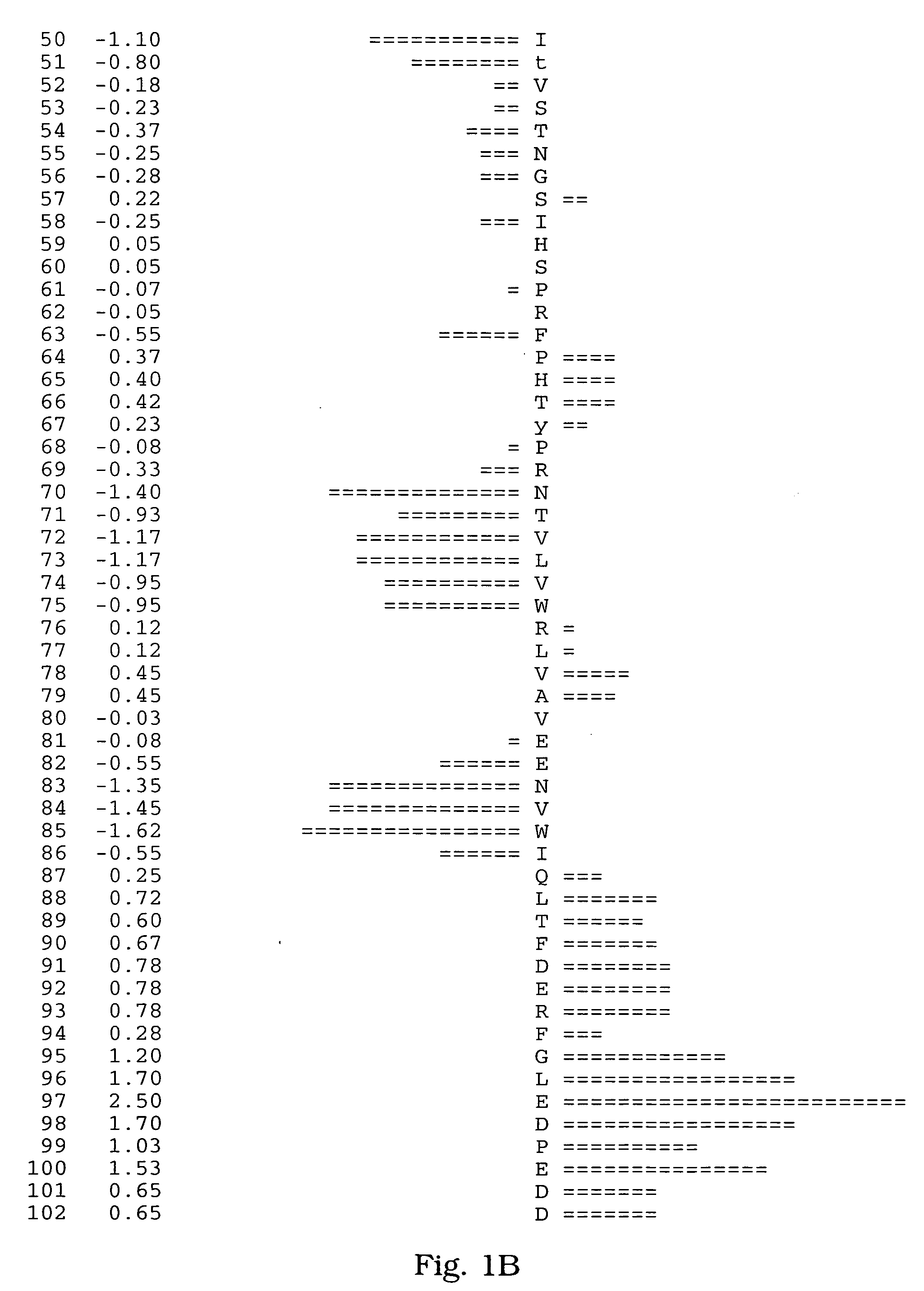
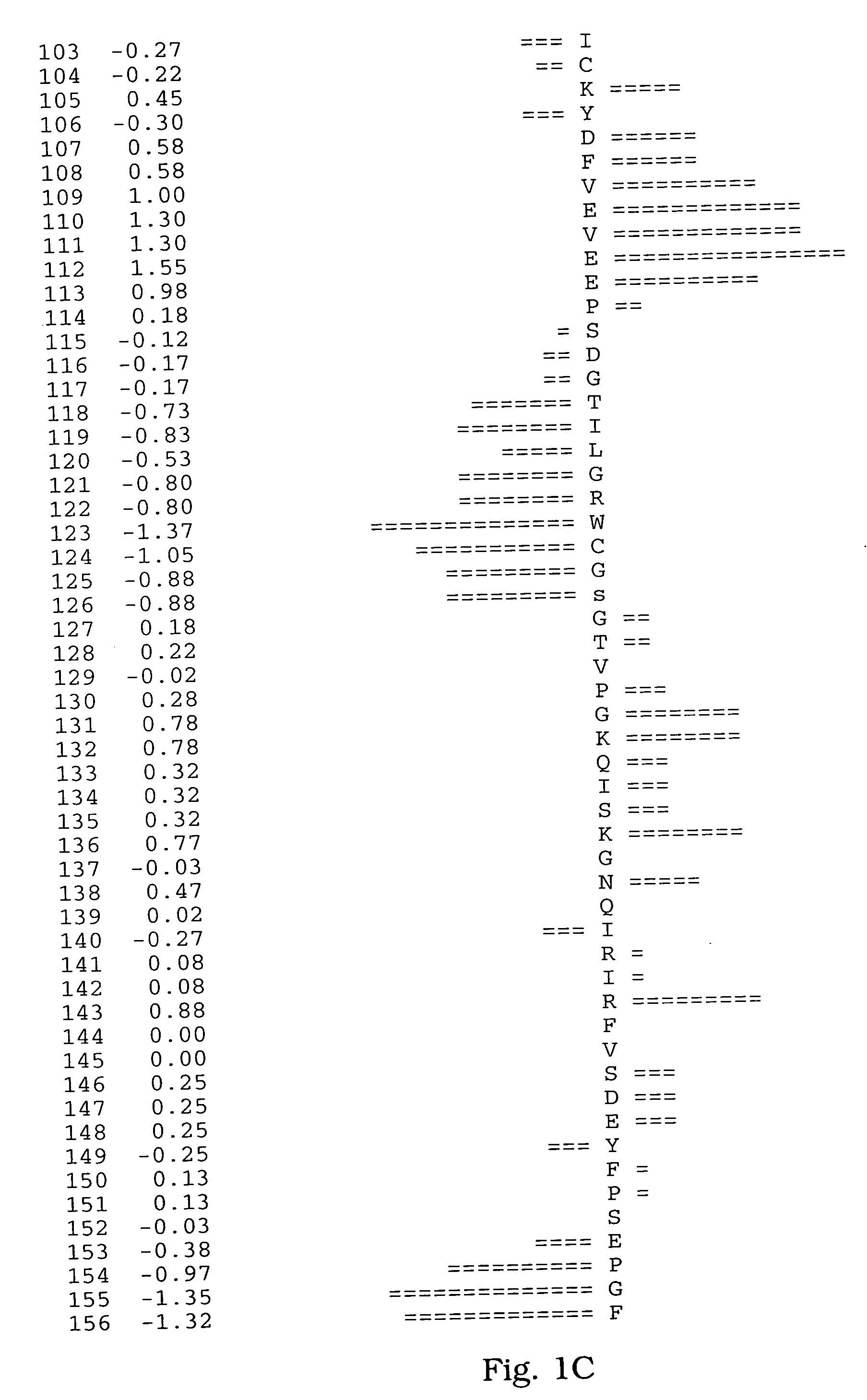
[0065] A human salivary gland library was screened for a full-length clone of zvegf3 by PCR. This library was an arrayed library representing 9.6×105 clones made in the vector pZP5x. The vector pZP5x is the same as vector pZP-9 (deposited with American Type Culture Collection, 10801 University Blvd., Manassas, Va. under Accession Number 98668), but contains a cytomegalovirus promoter instead of a metallothionein promoter between the Asp718 and BamHI sites. The plasmid thus comprises a dihyrofolate reductase gene under control of the SV40 early promoter and SV40 polyadenylation site, and a cloning site to insert the gene of interest under control of the CMV promoter and the human growth hormone (hGH) gene polyadenylation site. The working plate containing 80 pools of 12,000 colonies each was screened by PCR using oligonucleotide primers ZC19,045 (SEQ ID NO:6) and ZC19,047 (SEQ ID NO:7) with an annealing temperature of 60° C. for 35 cycles. There were two strong positives, pools 58 (T...
example 2
[0067] A PCR panel was screened for mouse zvegf3 DNA. The panel contained 8 cDNA samples from brain, bone marrow, 15-day embryo, testis, salivary gland, placenta, 15-day embryo (Clontech Laboratories), and 17-day embryo (Clontech Laboratories) libraries.
[0068] PCR mixtures contained oligonucleotide primers ZC21,222 (SEQ ID NO:9) and ZC21,224 (SEQ ID NO:10). The reaction was run at an annealing temperature of 66° C. with an extension time of 2 minutes for a total of 35 cycles using Ex Taq™ DNA polymerase (PanVera, Madison, Wis.) plus antibody. DNA samples found to be positive for zvegf3 by PCR and confirmed by sequencing included mouse 15-day embryo library total pool cDNA, mouse 15-day embryo (Clontech Laboratories) and 17-day embryo (both obtained from Clontech Laboratories), mouse salivary gland library total pool cDNA, and mouse testis library total pool cDNA. Fragments of about 600 bp from each of the mouse 15-day embryo library total pool cDNA, mouse 15-day embryo mcDNA, and m...
example 3
[0072] A mammalian cell expression vector for the growth factor domain of zvegf3 was constructed by joining the zvegf3 fragment to a sequence encoding an optimized t-PA secretory signal sequence (U.S. Pat. No. 5,641,655) in the linearized pZMP11 vector downstream of the CMV promoter. The plasmid pZMP11 is a mammalian expression vector containing an expression cassette having the CMV immediate early promoter, a consensus intron from the variable region of mouse immunoglobulin heavy chain locus, Kozak sequences, multiple restriction sites for insertion of coding sequences, a stop codon, and a human growth hormone terminator. The plasmid also contains an IRES element from poliovirus, the extracellular domain of CD8 truncated at the C-terminal end of the transmembrane domain, an E. coli origin of replication, a mammalian selectable marker expression unit having an SV40 promoter, enhancer and origin of replication, a DHFR gene, the SV40 terminator, and the URA3 and CEN-ARS sequences requ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com