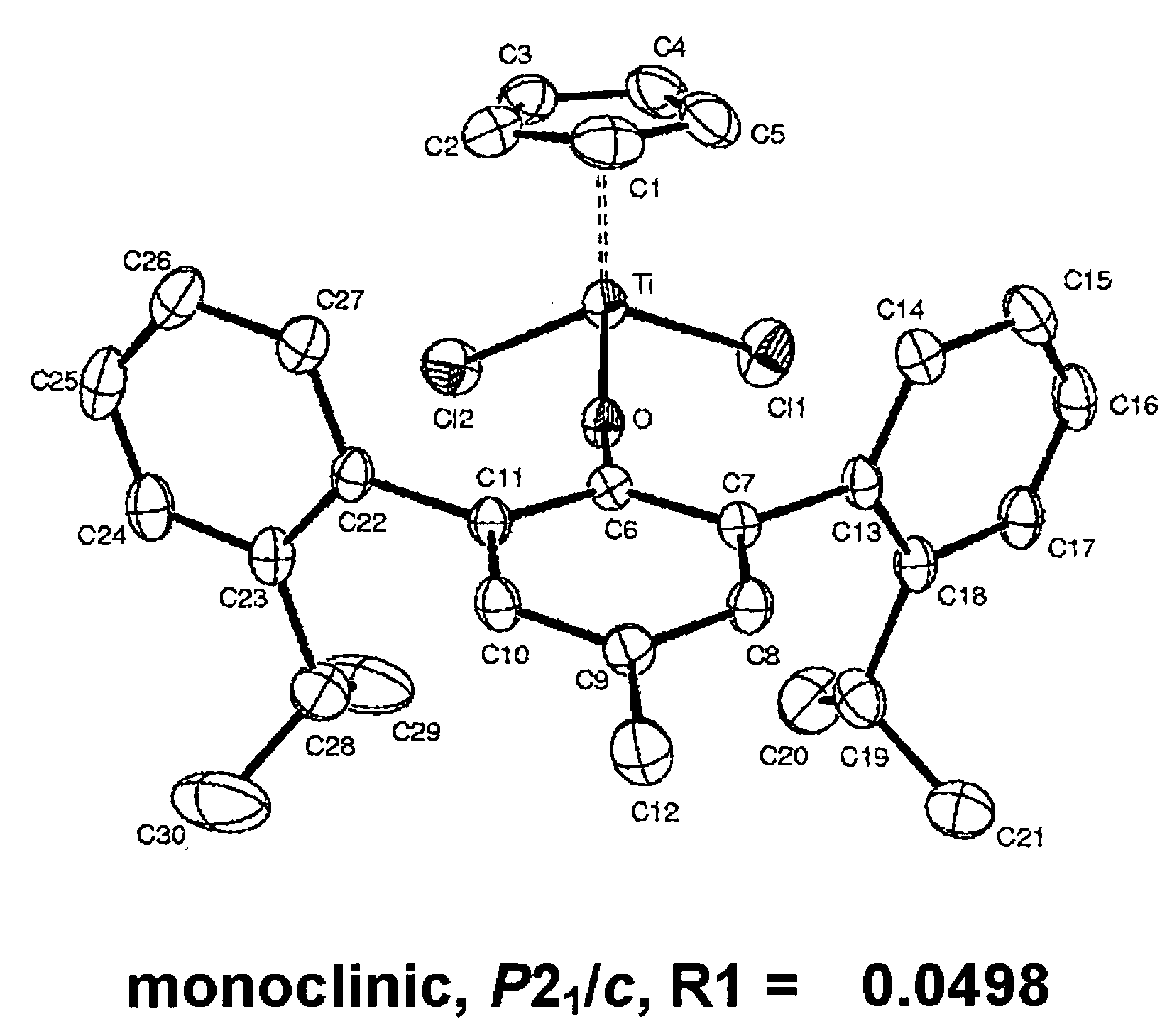
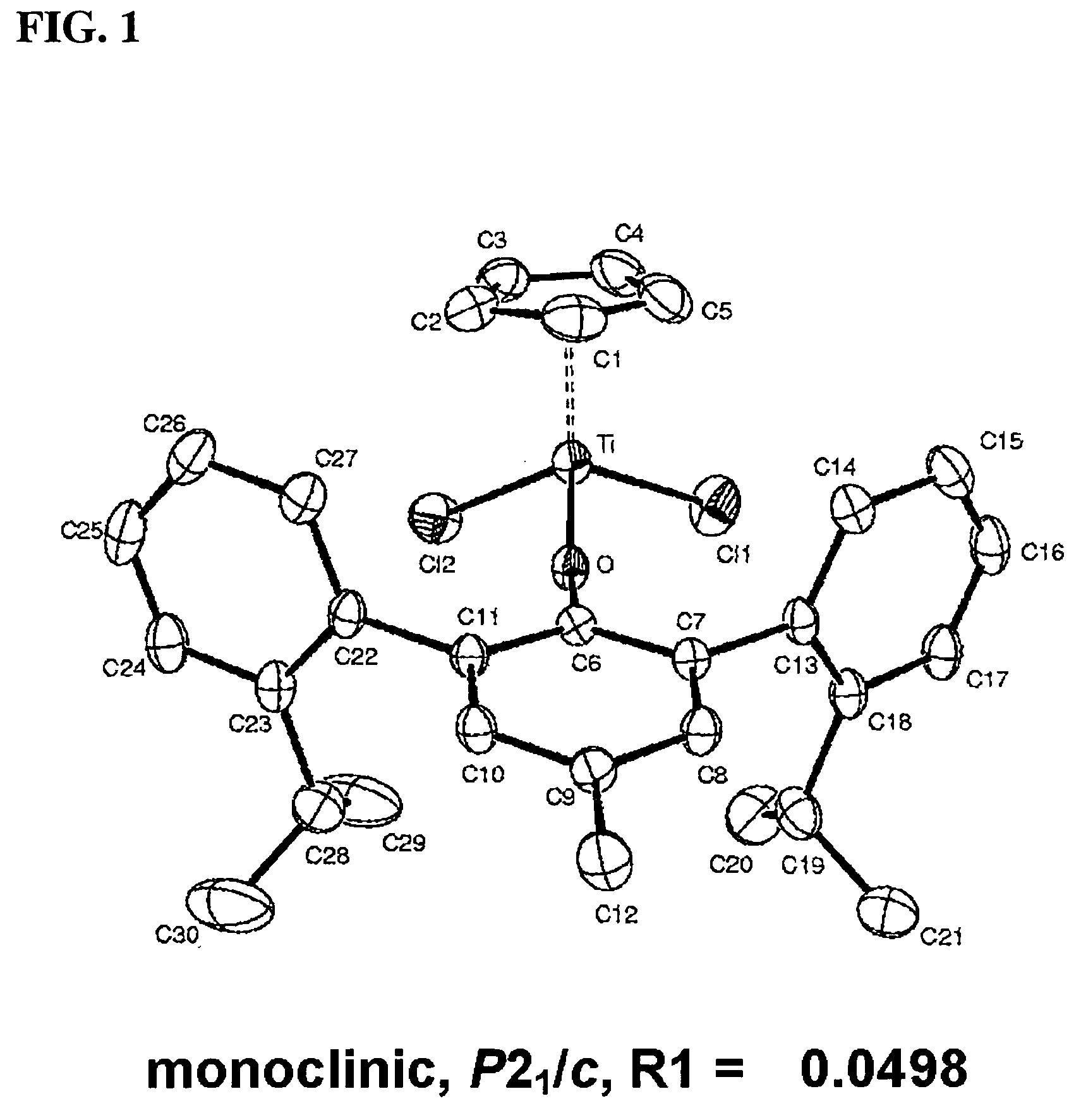
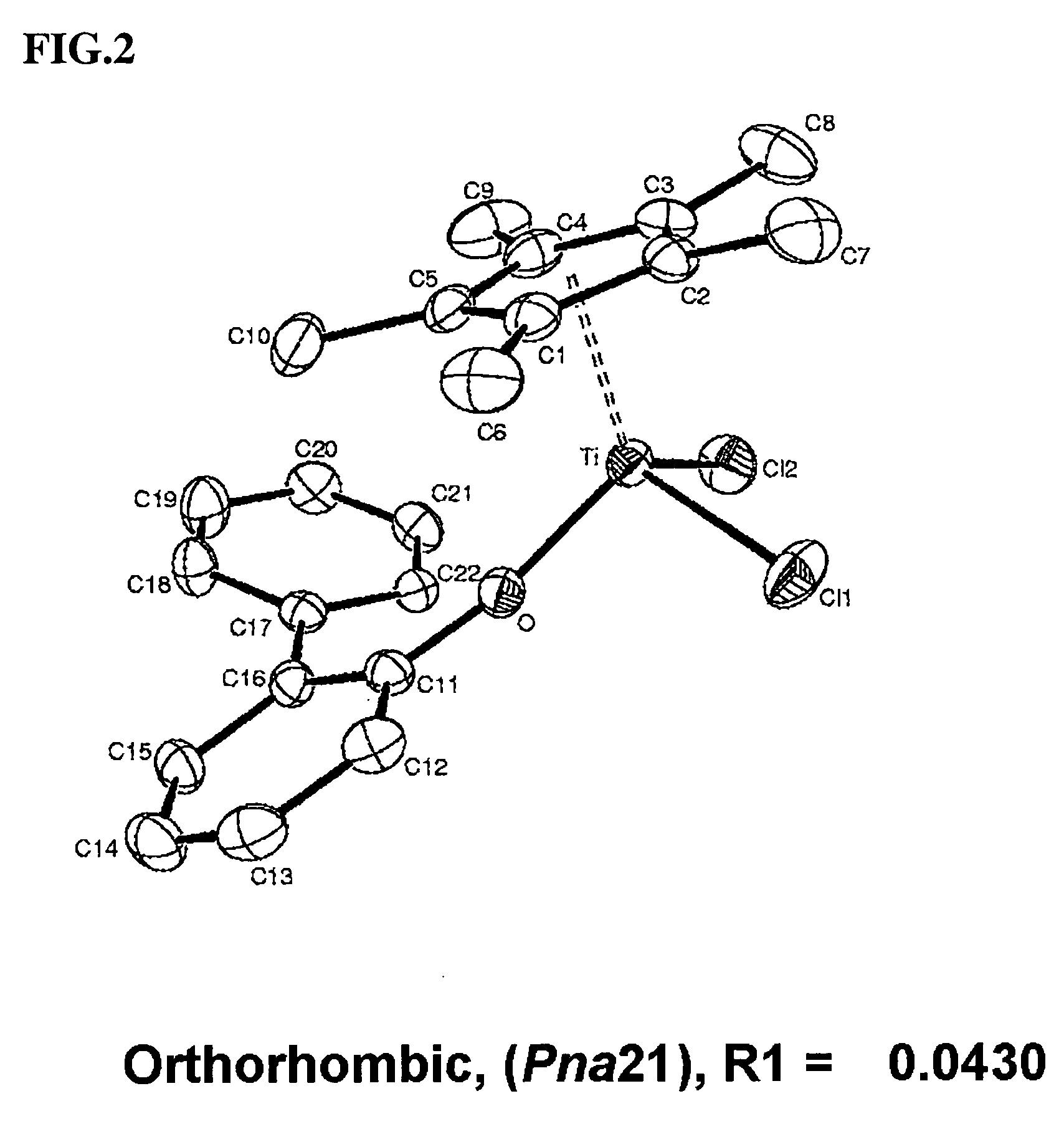
Arylphenoxy catalyst system for producing ethylene homopolymer or copolymers of ethylene and alpha-olefins
a technology of arylphenoxy catalyst and ethylene, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, chemical/physical process, etc., can solve the problems of difficult to produce a polymer having and unsuitable for producing a high molecular weight polymer having a weight average molecular weight of 100,000, etc., to achieve excellen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
Synthesis of 4-methyl-2,6-bis(2′-isopropylphenyl)phenol
[0070] A mixed solution of 1 mL of water and 4 mL of dimethoxyethane was added into a flask into which 2,6-dibromo-4-methylanisole (400 mg, 1.43 mmol), 2-isopropylphenylboronic acid (720 mg, 4.39 mmol), palladium acetate (14 mg, 0.062 mmol), triphenylphosphine (60 mg, 0.23 mmol), and potassium phosphate (940 mg, 4.43 mmol) were already added, and then refluxed at normal temperature for 6 hours. After cooling to normal temperature, an ammonium chloride aqueous solution (5 mL) and 10 mL of diethylether were added thereto to separate an organic layer and extract residues using diethylether. The separated organic layer was dried with magnesium sulfate and volatile materials were then removed to produce 670 mg of grey 4-methyl-2,6-bis(2′-isopropylphenyl)anisole solid. The anisole thus produced was dissolved in 5 mL of methylene chloride without separate purification, 3 mL of boron tribromide (1 M methylene chloride solution) was dro...
example 1
[0074] 300 mL of n-heptane was added into a stainless steel reactor which was purged with nitrogen after sufficient drying and had a volume of 500 m / L, and 0.5 mL of triisobutylaluminum (Aldrich) (200 mM n-heptane solution) was added thereto. The temperature of the reactor was then increased to 140° C., and, subsequently, 0.2 mL of (dichloro)(pentamethylcyclopentadienyl)(4-methyl-2,6-bis(2′-isopropylphenyl)phenoxy)titanium(IV) (5 mM toluene solution), produced according to preparation example 1, and 0.3 mL of triphenylmethylinium tetrakis(pentafluorophenyl)borate (99%, Boulder Scientific) (5 mM toluene solution) were sequentially added thereto. Ethylene was then injected into the reactor until the pressure in the reactor was 30 atm, and was continuously fed for polymerization. 10 min after the reaction started, 10 mL of ethanol (including 10 vol % hydrochloric acid aqueous solution) were added to finish the polymerization, agitation was conducted for 4 hours along with 1500 mL of ad...
example 2
[0075] 15 mL of 1-octene were injected into a reactor which was the same as in example 1, and polymerization was then conducted through the same procedure as in example 1 except that 0.3 mL of (dichloro)(pentamethylcyclopentadienyl)(4-methyl-2,6-bis(2′-isopropylphenyl)phenoxy)titanium(IV) (5 mM toluene solution) and 0.45 mL of triphenylmethylinium tetrakis(pentafluorophenyl)borate (Boulder Scientific) (5 mM toluene solution) were added after 0.75 mL of triisobutylaluminum (Aldrich) (200 mM n-heptane solution) were added. 4.0 g of dried polymer was obtained. The weight average molecular weight was 175,000 and a molecular weight distribution was 5.91, which were determined through gel chromatography analysis. The melt index was 0.12 g / 10 min, the melting point of the polymer was 114.7° C., the density was 0.9215 g / cc, and the content of 1-octene was 7.8 wt %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com