Pharmaceutical Preparation and Method of Treatment of Human Malignancies with Arginine Deprivation
a technology of arginine deprivation and pharmaceutical preparation, which is applied in the direction of drug compositions, enzyme stabilisation, peptide/protein ingredients, etc., can solve the problems of inability to treat cancer in vivo with arginine depletion, failure of previous attempts to deplete arginine with various physical methods or arginine degrading enzymes, etc., to achieve “adequate arginine deprivation” and increase the stability of enzym
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
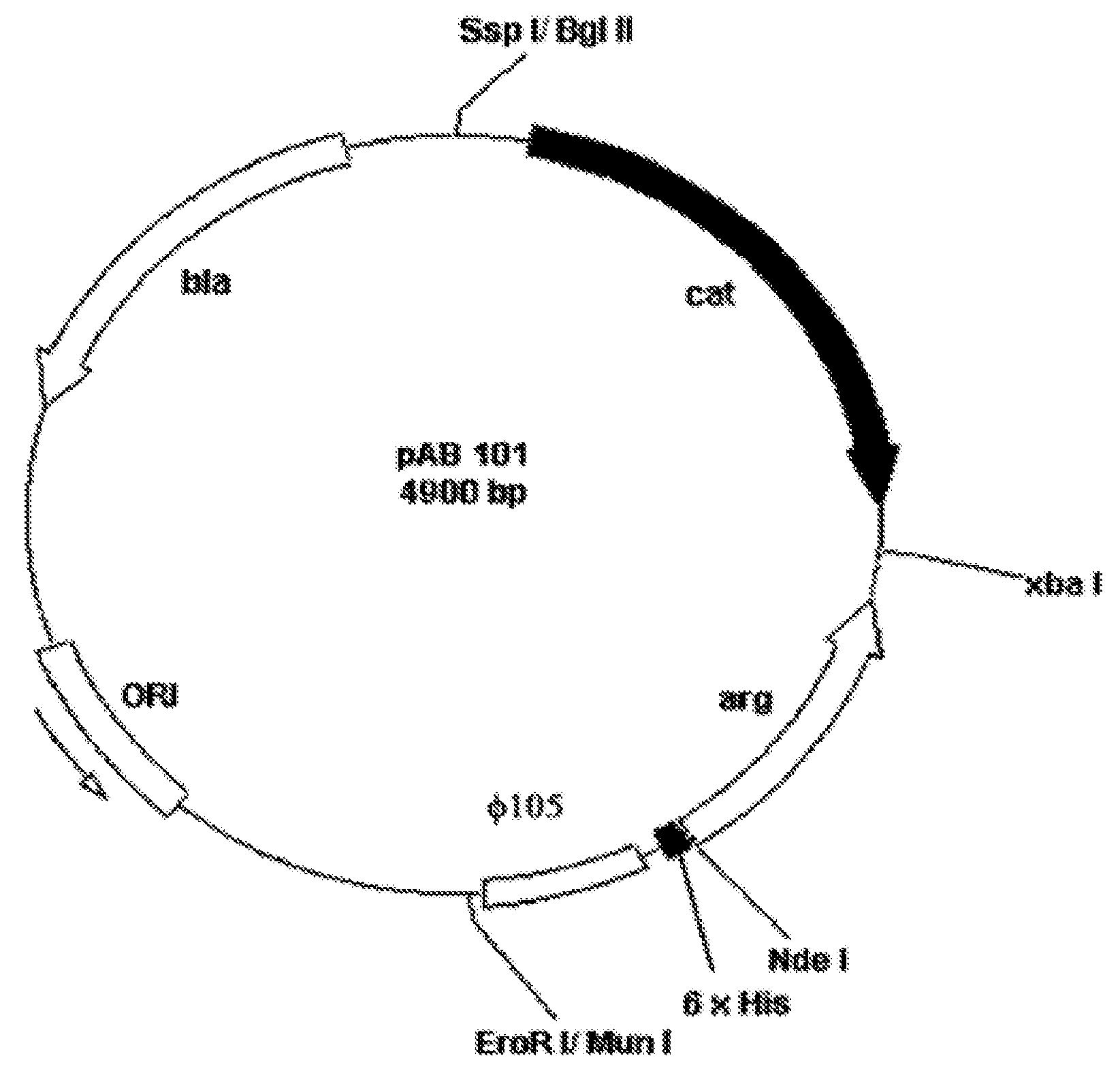
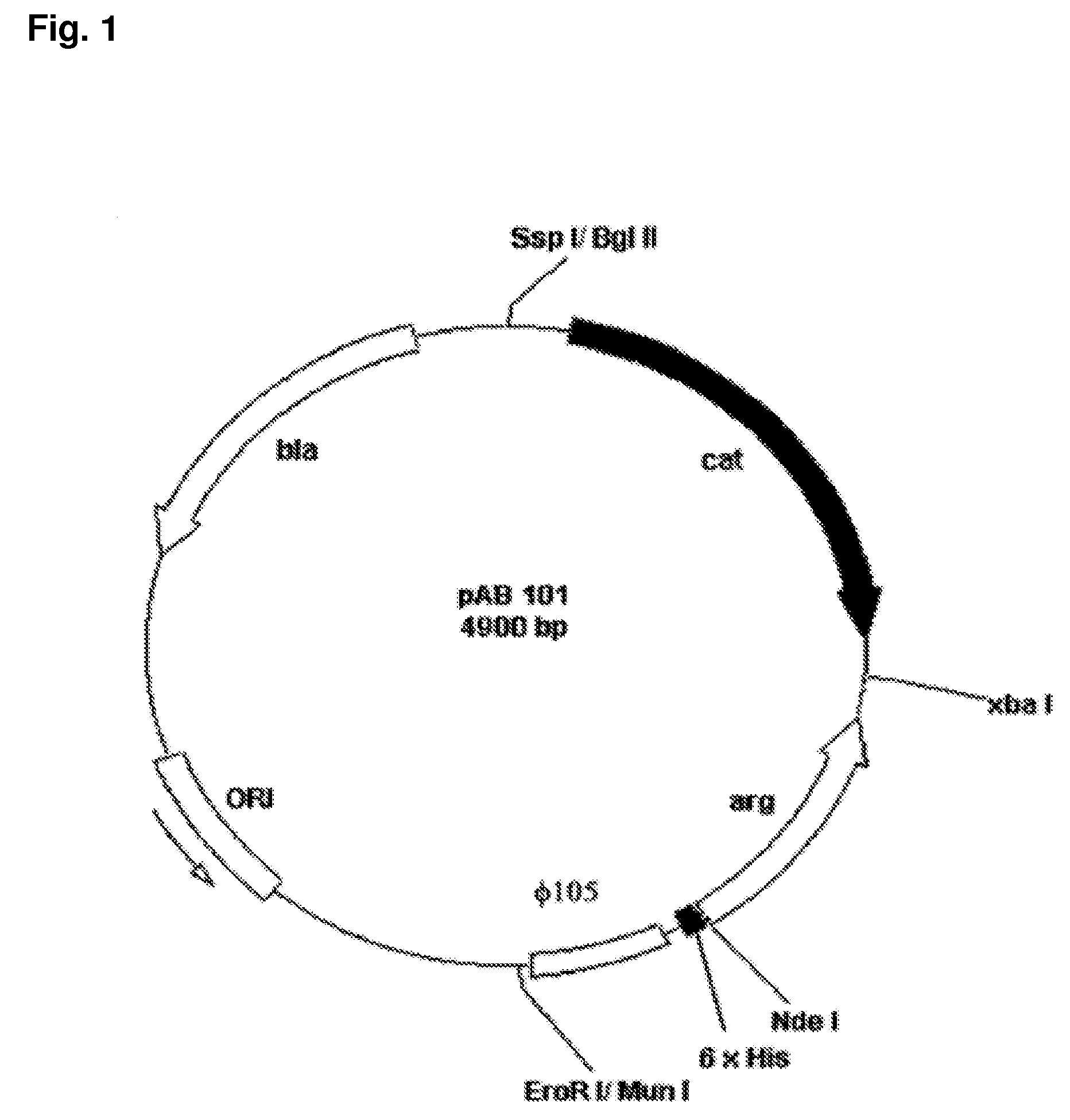
Construction of the Recombinant Strain LLC101
[0066](a) Isolation of the Gene Encoding Human Arginase I
[0067]The gene sequence of human Arginase I was published in 1987 (Haraguchi, Y. et al., 1987, Proc. Natl. Acad. Sci. 84, 412-415) and primers designed therefrom. Polymerase chain reaction (PCR) was performed to isolate the gene encoding a human Arginase using the Expand High Fidelity PCR System Kit (Roche). Primers Arg1 (5′-CCAAACCATATGAGCGCCAAGTCCAGAACCATA-3′) (SEQ ID NO: 5) and Arg2 (5′-CCAAACTCTAGAATCACATTTTTTGAATGACATGGACAC-3′) (SEQ ID NO: 6), respectively, were purchased from Genset Singapore Biotechnology Pte Ltd. Both primers have the same melting temperature (Tm) of 72 degree C. Primer Arg1 contains a NdeI restriction enzyme recognition site (underlined) and primer Arg2 contains a XbaI site (underlined). These two primers (final concentration 300 nM of each) were added to 5 μl of the human liver 5′-stretch plus cDNA library (Clontech) in a 0.2-ml micro-tube. DNA polymerase...
example 2a
Batch Fermentation in a 2-Liter Fermentor
[0076]The B. subtilis LLC101 strain is maintained on a Nutrient Agar (beef extract 1 g / L, peptone 10 g / L, NaCl 5 g / L and agar 20 g / L) plate, supplemented with 5 mg / L of chloramphenicol. To prepare the innoculum for batch and fed-batch fermentation, a few colonies of the aforementioned strain were transferred from a freshly prepared Nutrient Agar plate into two 1-L flasks, each containing 80 mL of fermentation medium containing glucose 5 g / L, tryptone 10 g / L, yeast extract 3 g / L, sodium citrate 1 g / L, KH2PO4 1.5 g / L, K2HPO4 1.5 g / L, and (NH4)2SO4 3 g / L. The bacterial cell culture was cultivated at 37° C. and pH 7.0 on an orbital shaker rotating at 250 r.p.m. The cultivation was terminated when OD600 nm reached 5.5-6.0 at about 9-11 h growth time. Then the 160-mL culture broth was introduced into the 2-L fermentor containing 1440-mL fermentation medium (glucose 5 g / L, tryptone 10 g / L, yeast extract 3 g / L, sodium citrate 1 g / L, KH2PO4 1.5 g / L, K...
example 2b
Fed-Batch Fermentation in a 2-Liter Fermentor
[0077]The Fed-batch fermentation was carried out at 37 degree C., pH 7.0 and dissolved oxygen 20% air saturation. The inoculation procedure was similar to that of the batch fermentation described in Example 2A. Initially, the growth medium was identical to that used in the batch fermentation described in Example 2A. The feeding medium contained 200 g / L glucose, 2.5 g / L MgSO4.7H2O, 50 g / L tryptone, 7.5 g / L K2HPO4 and 3.75 g / L KH2PO4. The medium feeding rate was controlled with the pH-stat control strategy. In this strategy, the feeding rate was adjusted to compensate the pH increase caused by glucose depletion. This control strategy was first implemented when the glucose concentration decreased to a very low level at about 4.5-h fermentation time. If pH>7.1, 4 mL of feeding medium was introduced into the fermentor. Immediately after the addition of glucose, the pH value would decrease below 7.1 rapidly. After approximate 10 min, when the g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diffusion distance | aaaaa | aaaaa |
| OD | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com