[0015]The present invention overcomes the drawbacks of previously-known
fluid management systems for treating ascites by providing a
fluid management system that automatically and autonomously removes ascites accumulations with little patient involvement. The fluid
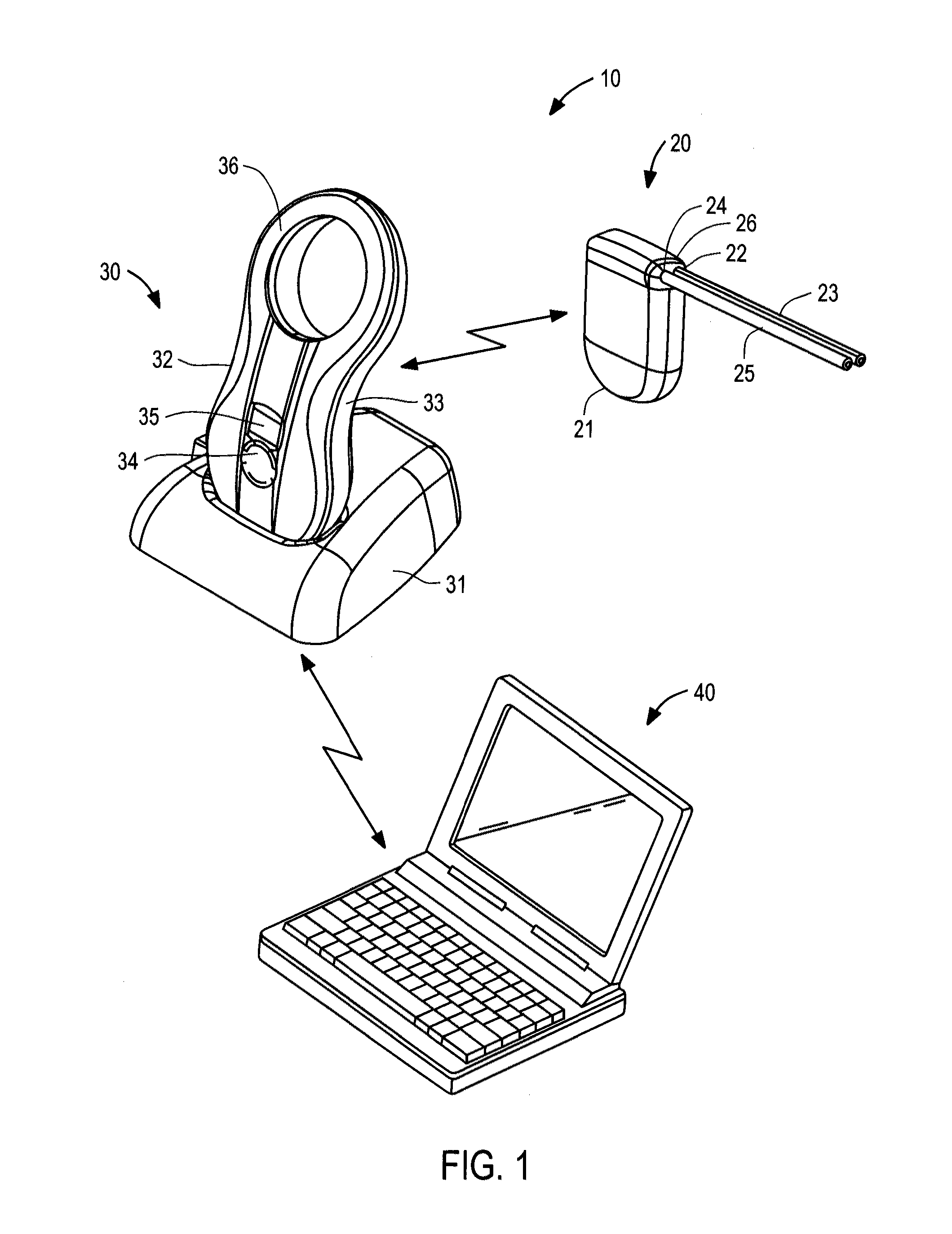
management system of the present invention preferably comprises an implantable device including a pump, a controller, a battery and a
transceiver; a charging and communication system configured to periodically charge the battery of, and communicate with, the implantable device; and
monitoring and control software, suitable for use with a conventional
personal computer, for configuring and controlling operation of the implantable device and charging and communication system. Preferably, the monitoring and
control software is available only to the treating physician, such that the patient generally interacts with the implantable device only via the charging and communication system for purposes of recharging the implantable device. In accordance with one aspect of the present invention, the implantable device is configured to pump fluid in small increments, at relatively high flow rates, during predetermined times of the day to achieve a target volume, and further is configured to periodically alter the pump position to reduce the risk of clogging of the implantable device during non-pumping intervals. The pump also may be programmed to perform a rapid sequence of backward and forward movements if a blockage is detected, thereby clearing the blockage. Additionally, the fluid
management system may include one or more sensors configured to detect indicia of the onset of infection, e.g., an increase in temperature,
respiratory rate, or the
viscosity of
ascitic fluid, and one or more alarms configured to indicate to the physician a prediction or detection of infection based on the output(s) of those sensors.
[0017]In one preferred embodiment, the implantable device includes an electrically-driven mechanical
gear pump configured for
subcutaneous implantation. The pump has an inlet port coupled to an inflow
catheter and an outlet port coupled to a bladder
catheter. In accordance with one aspect of the present invention, the pump employs a pair of floating gears that function as a
positive displacement pump, wherein a driving gear is coupled to a splined shaft of an
electric motor to minimize
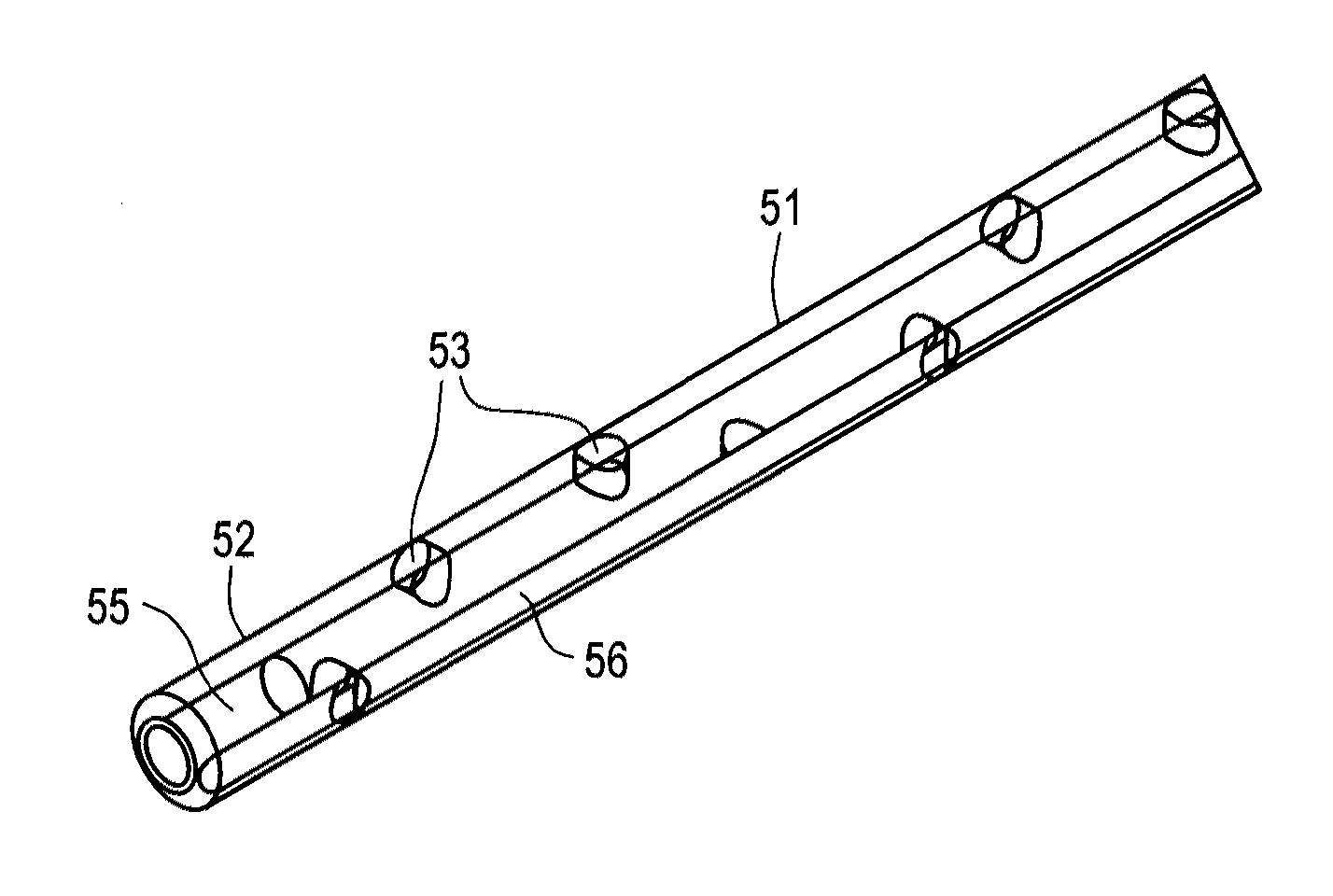
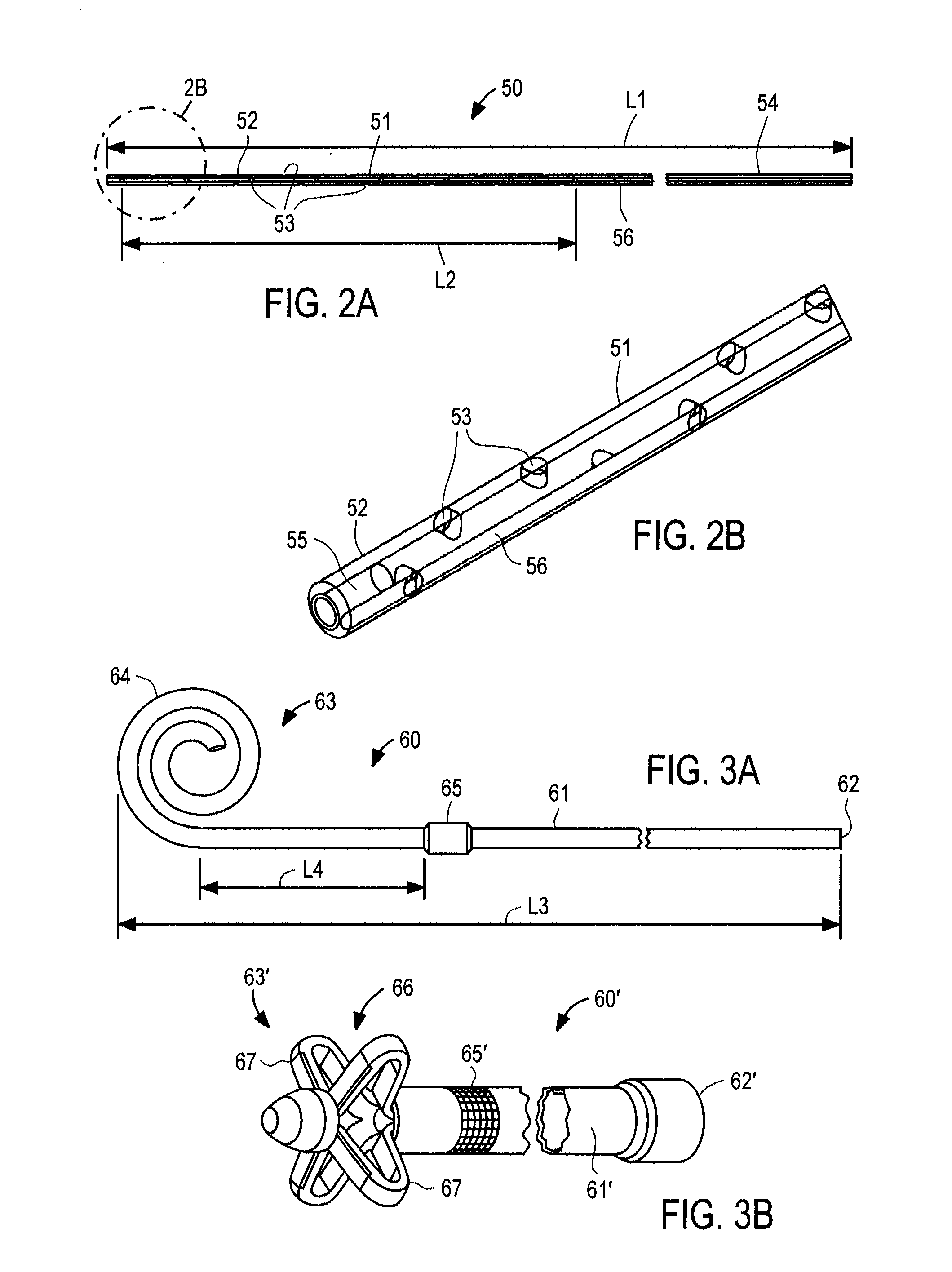
power consumption arising due to manufacturing variations or shaft eccentricity. The inflow
catheter comprises a tube having a first end configured to be coupled to the pump inlet and a second end configured to be positioned in a selected cavity, e.g.,
peritoneum, pleura or pericardial sac. The second end of the inflow catheter includes a plurality of through-wall apertures that permit fluid accumulating to pass into the catheter. The bladder or outflow catheter comprises a tube having a first end configured to be coupled to the pump and a second end configured to be inserted through the wall of, and fixed within, a patient's bladder. Alternatively, for treating pleural or pericardial effusions, the second end may be configured for placement in the peritoneal cavity, rather than the bladder. The fluid circuit further includes sensors arranged to monitor
ambient pressure, pressure at the pump inlet, pressure at the pump outlet, pressure in the bladder (or peritoneal cavity, if this is used as a sink), and optionally the temperature of the
ascitic fluid and the
respiratory rate of the patient. The inflow and outflow catheters include connectors configured to reduce the risk of improper implantation.
[0018]The implantable device further comprises a controller, packaged together with the pump,
electric motor, battery, charging coil, and radio
transceiver within a
low volume sealed housing. The controller is coupled to the pump motor, battery, transceiver and a plurality of sensors to continually monitor pressure, temperature,
humidity, charge status, pump status, patient movement and other environmental and system related parameters. The controller preferably comprises a processor, nonvolatile memory for storing
firmware,
implant identification information, and system and
environmental data, and
volatile memory that serves as a buffer for computations and instructions during execution and
firmware updating. The pump motor is configured for extended use and low
power consumption, and preferably includes
Hall effect sensors for position sensing and to determine the direction of rotation (and correspondingly, flow and
fluid viscosity). The battery preferably is a long-lasting
lithium-
ion or
lithium polymer battery that is coupled to an
inductive charging circuit, thereby enabling the battery to be recharged using the external charging and communication system. A
radio frequency transceiver preferably is employed in the device for transmitting
system information to, and receiving information from, the external charging and communication system, including system performance data, commands, and
firmware upgrades. All of the foregoing components preferable are disposed within the housing, which further includes a filler having a
low permeability for water, thereby reducing infiltration of
moisture into the housing.
[0021]It is contemplated that the system of the present invention may avoid difficulties typically associated with the previously-known apparatus and methods for addressing ascites. It is expected, for instance, that the system and methods of the present invention will enable small quantities of
peritoneal fluid to be moved to the bladder without the inconvenience and complications generally associated with use of pharmaceuticals or paracenteses. In particular, because the apparatus and methods of the present invention avoid repeated, periodic removal of large quantities of fluid, as occurs with paracenteses, the tendency to generate additional ascites to offset the removed fluid will be reduced. These effects in turn are expected to obviate the need to infuse
plasma expanders, such as
human albumin, into the patient following
paracentesis, thereby resulting in significant
cost savings to the patient and health care system. The prediction or detection of infection, particularly at an early stage of infection, further may improve patient outcomes and reduce the need for more expensive treatments. Finally, the apparatus and methods of the present invention are expected to provide improved
quality of life for chronic ascites patients, allowing such patients to pursue less sedentary lifestyles than would otherwise be possible, and encouraging better compliance with medically-directed dietary and
exercise regimes.
 Login to View More
Login to View More  Login to View More
Login to View More