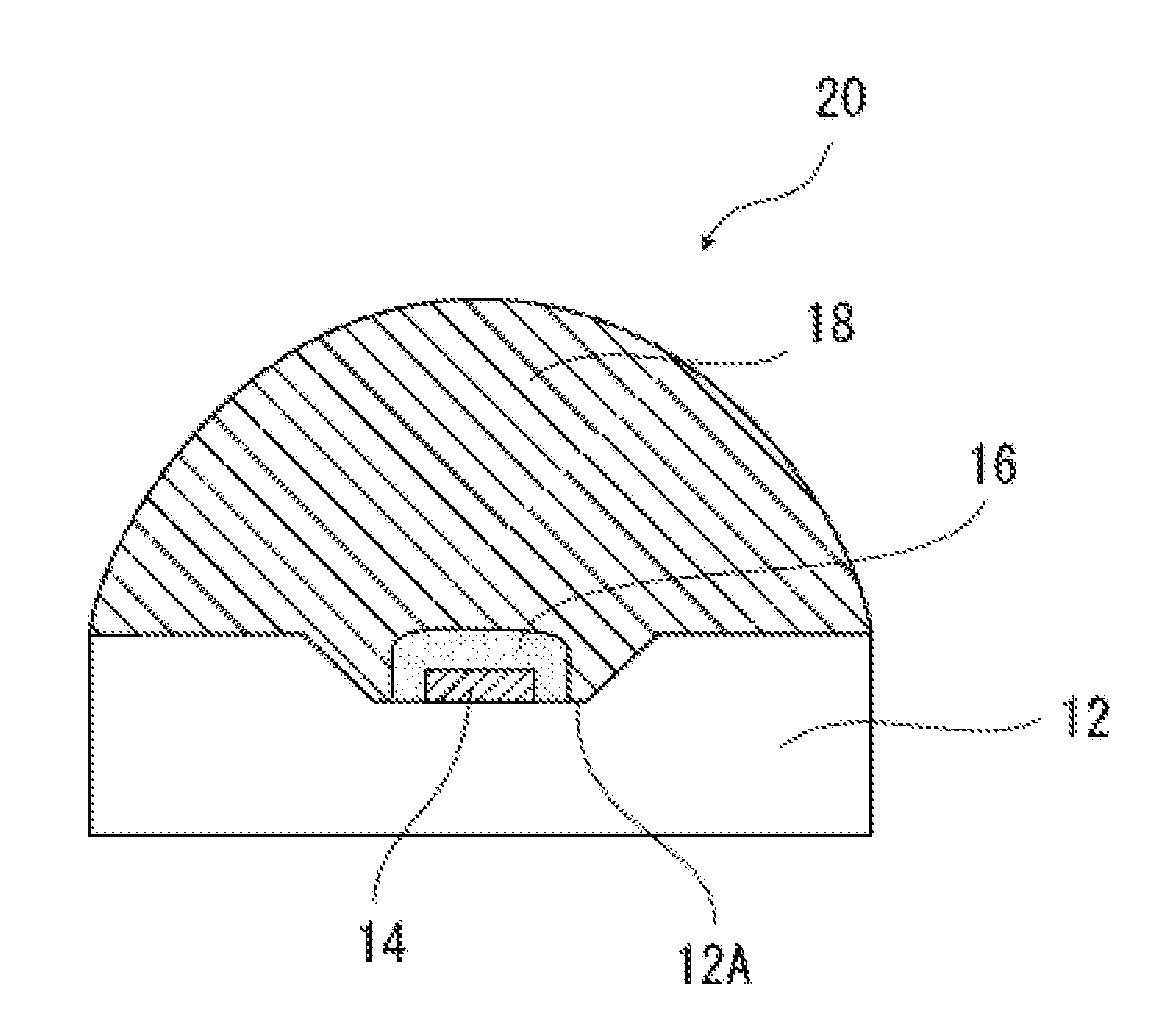
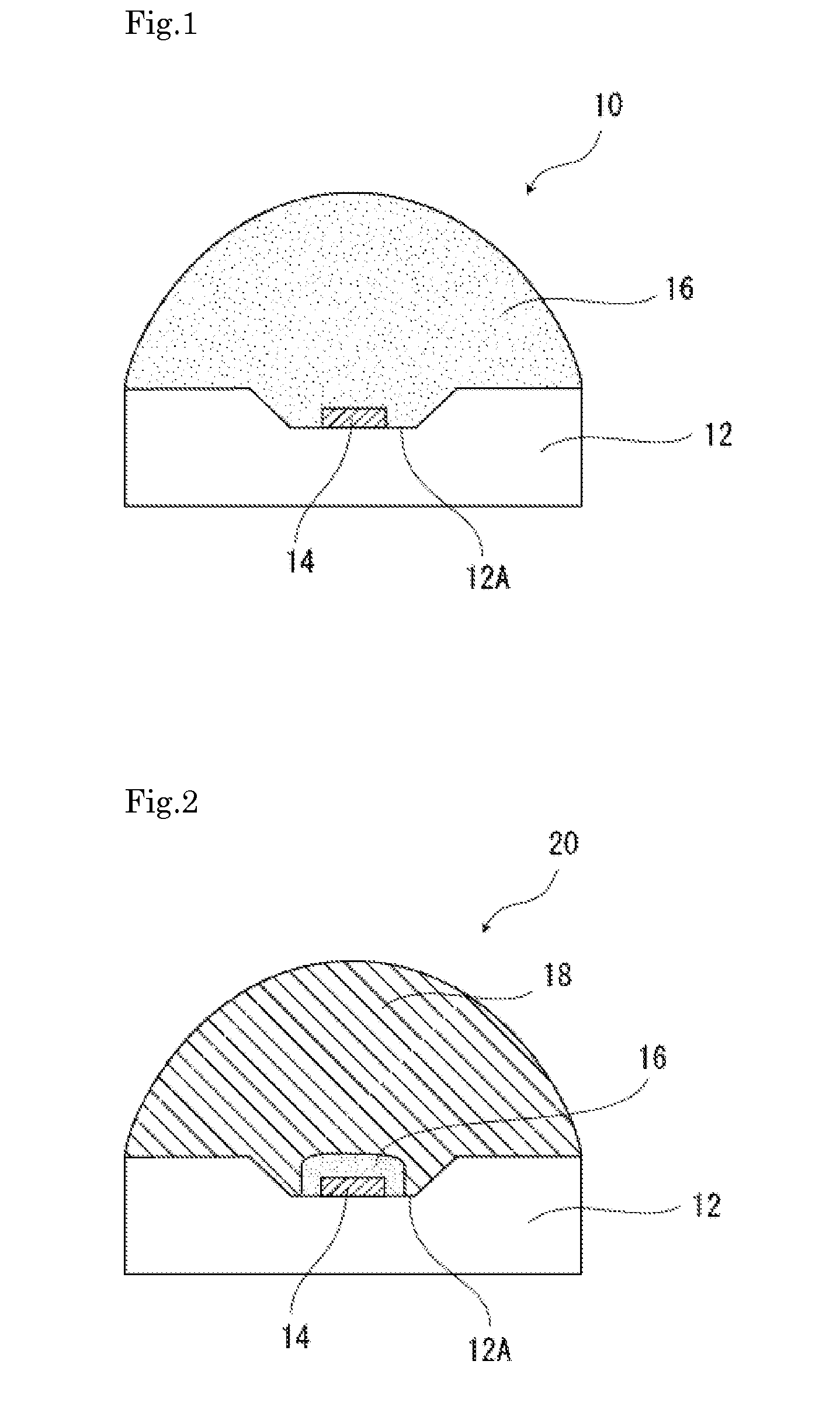
Surface-modified metal oxide particle material, dispersion liquid, silicone resin composition, silicone resin composite body, optical semiconductor light emitting device, lighting device, and liquid crystal imaging device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0153]The present invention is hereunder specifically described with reference to the following Examples and Comparative Examples, but it should not be construed that the present invention is limited to these Examples.
[0154]With respect to the present Examples, various measurements and evaluations were performed in the following manners.
(Average Primary Particle Diameter of Metal Oxide Particle)
[0155]An average primary particle diameter of a metal oxide particle was defined as a Scherrer's diameter which is obtained by calculation from a half-value width of an X-ray diffraction peak. This is because so long as the primary particle diameter is a nanometer size, a possibility that one particle is constituted of plural crystallites is low, so that the average primary particle diameter and the Scherrer's diameter are substantially equal to each other.
(Transmittance of Silicone Resin Composite)
[0156]A transmittance of a silicone resin composite was measured by using a composite (thicknes...
example a1
Fabrication of Zirconia Particle
[0164]To a zirconium salt solution of 2,615 g of zirconium oxychloride octahydrate dissolved in 40 L (liters) of pure water, dilute ammonia water of 344 g of 28% ammonia water dissolved in 20 L of pure water was added while stirring, thereby preparing a zirconia precursor slurry.
[0165]Subsequently, a sodium sulfate aqueous solution of 300 g of sodium sulfate dissolved in 5 L of pure water was added to this slurry while stirring. At this time, the addition amount of sodium sulfate was 30% by mass relative to a zirconia conversion value of a zirconium ion in the zirconium salt solution.
[0166]Subsequently, this mixture was dried in the air at 130° C. for 24 hours by using a dryer, thereby obtaining a solid.
[0167]Subsequently, this solid was pulverized by an automatic mortar and then baked in the air at 500° C. for one hour by using an electric furnace.
[0168]Subsequently, this baked material was put into pure water and stirred to make into a slurry form. ...
example a2
Fabrication of Zirconia Particle
[0175]A zirconia particle was fabricated in the same manner as that in Example A1.
(Fabrication of Surface-Modifying Material Containing Both a Phenyl Group and an Alkenyl Group)
Preparation of Surface-Modifying Material A: (CH2═CM(CH3)2SiO(SiO(C6H5)2)45Si(OC2H5)3
[0176]1.8 g of dimethyl vinyl silanol was dissolved in 60 mL of a tetrahydrofuran (THF) solvent in a nitrogen atmosphere, 1.2 g of n-butyl lithium dissolved in n-hexane was added dropwise at a temperature of 0° C. while stirring, and the contents were allowed to react with each other for 3 hours, thereby obtaining lithium dimethyl vinyl silanolate (see formula (A)).
[0177]Subsequently, a solution of 160.5 g of hexaphenyl cyclotrisiloxane dissolved in a THF solvent was added dropwise, and the contents were allowed to react with each other at a temperature of 0° C. for 12 hours, thereby obtaining lithium phenylvinyl organosilanolate (see formula (B)).
[0178]Subsequently, 3.6 g of chlorotriethoxysi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com