Use of dianhydrogalactitol or derivatives or analogs thereof for treatment of pediatric central nervous system malignancies
a technology of dianhydrogalactitol and derivatives, which is applied in the direction of x-ray/gamma-ray/particle irradiation therapy, heavy metal active ingredients, drug compositions, etc., can solve the problems of inability to meet preclinical testing and federal regulatory requirements for clinical evaluation, failure or disappointment of many compounds that have successfully met preclinical testing and clinical evaluation requirements, and chemical agents where in vitro and in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Activity of Dianhydrogalactitol to Against Medulloblastoma and Pediatric High Grade Glioma Cell Lines
[1549]Medulloblastoma (MB) is the most common malignant pediatric brain tumor, accounting for 15-30% of all childhood intracranial neoplasms. High grade gliomas (HGG) are much rarer in children than in adults, comprising only 5%-10% of childhood brain tumors. Although multidisciplinary treatment has improved the 5-year survival rates in children significantly, the prognosis for recurrent MB and HGG remains poor with median overall survival 6-methylguanine-DNA methyltransferase (MGMT), which is correlated with TMZ resistance, have been reported. Dianhydrogalactitol (VAL-083) is a structurally unique bi-functional alkylating agent causing DNA crosslinks at N7 position of guanine. VAL-083 readily crosses the blood brain barrier and has been shown to accumulate in brain tumor tissue. Furthermore, VAL-083 demonstrated clinical activity against MB and HGG in historical NCI-sponsored clinic...
example 2
Additional Applications for Use of Dianhydrogalactitol to Treat Central Nervous System Malignancies
[1562]Additional results for the use of dianhydrogalactitol to treat central nervous system malignancies, including glioblastoma multiforme, are provided below.
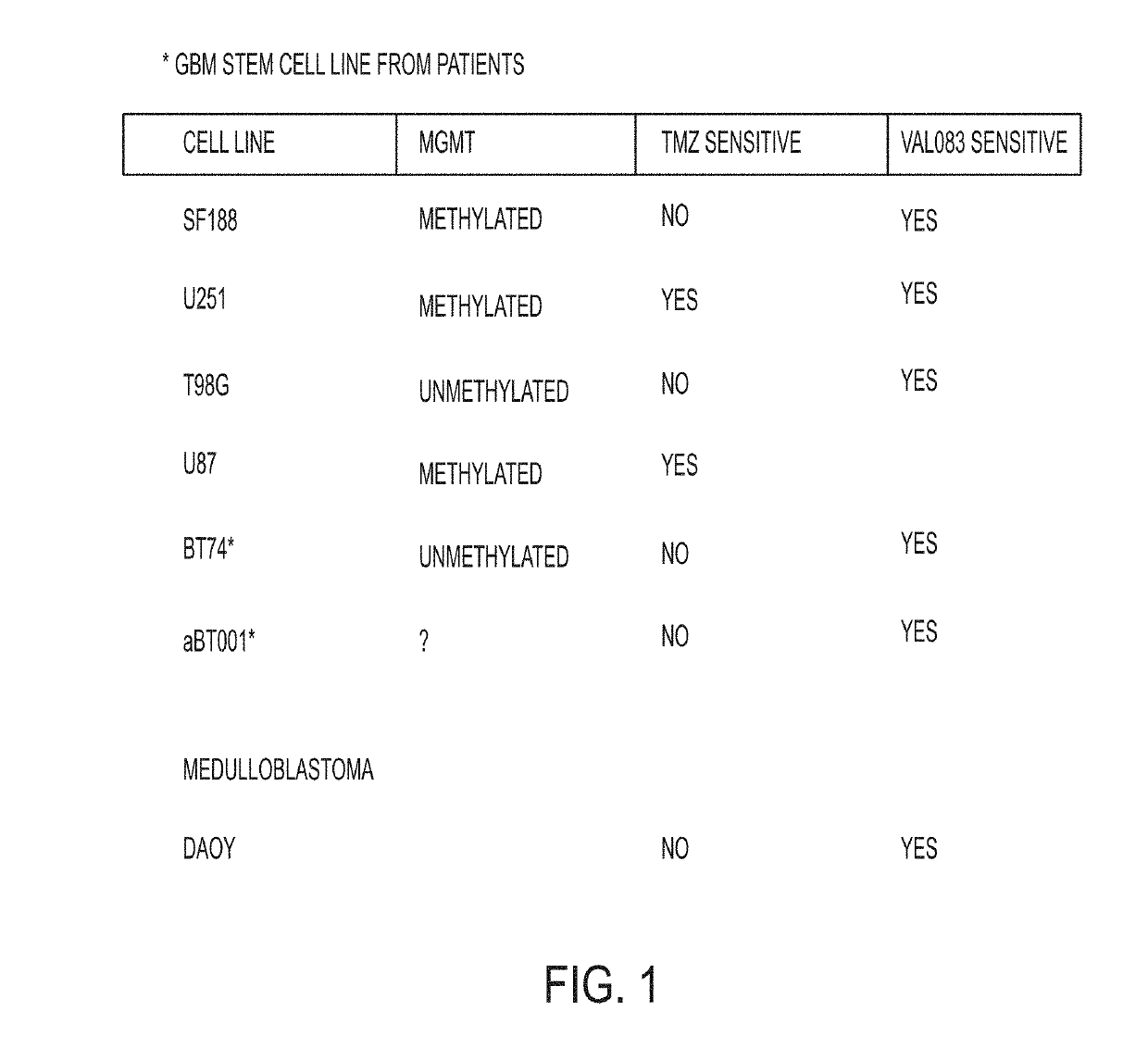
[1563]FIG. 1 is a table showing a summary of glioblastoma multiforme (GBM) models and their response to dianhydrogalactitol (VAL-083).
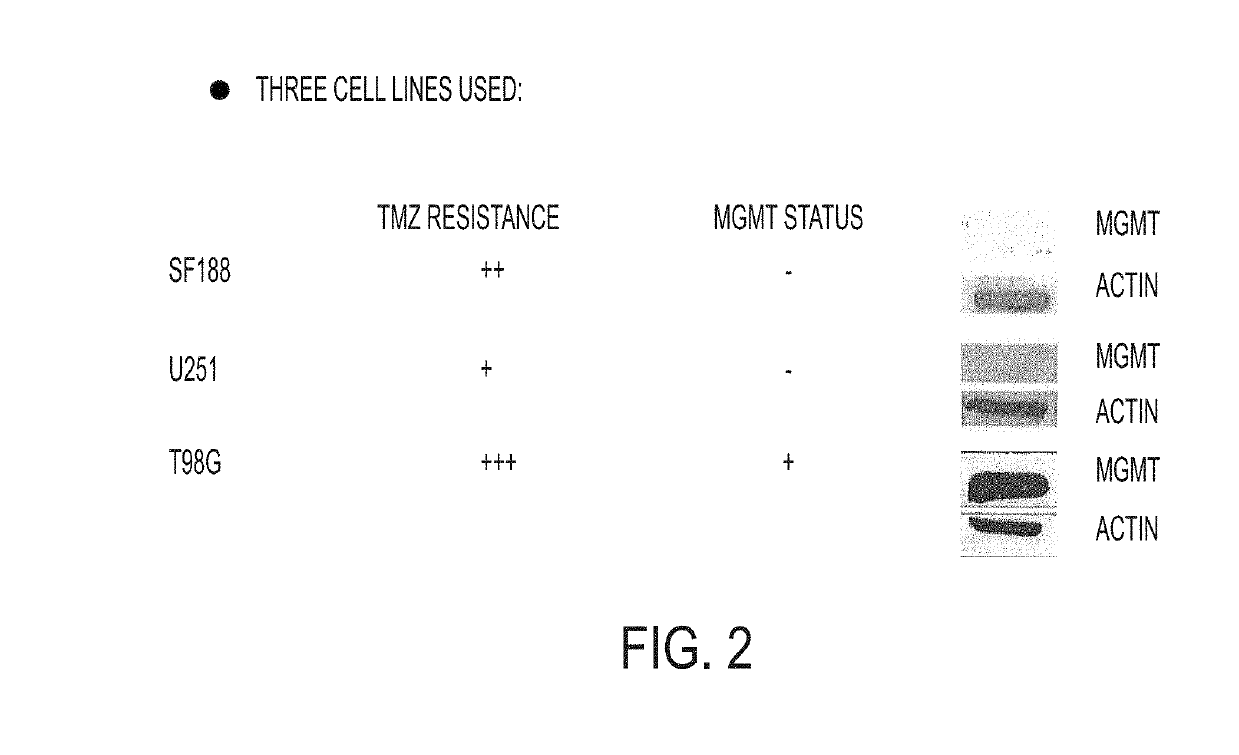
[1564]FIG. 2 shows the temozolomide (TMZ) resistance and MGMT status for three cell lines, SF188, U251, and T98G. SF188 is a pediatric high grade glioma cell line. In FIG. 2, the protein actin is used as a control. Western blots are shown.
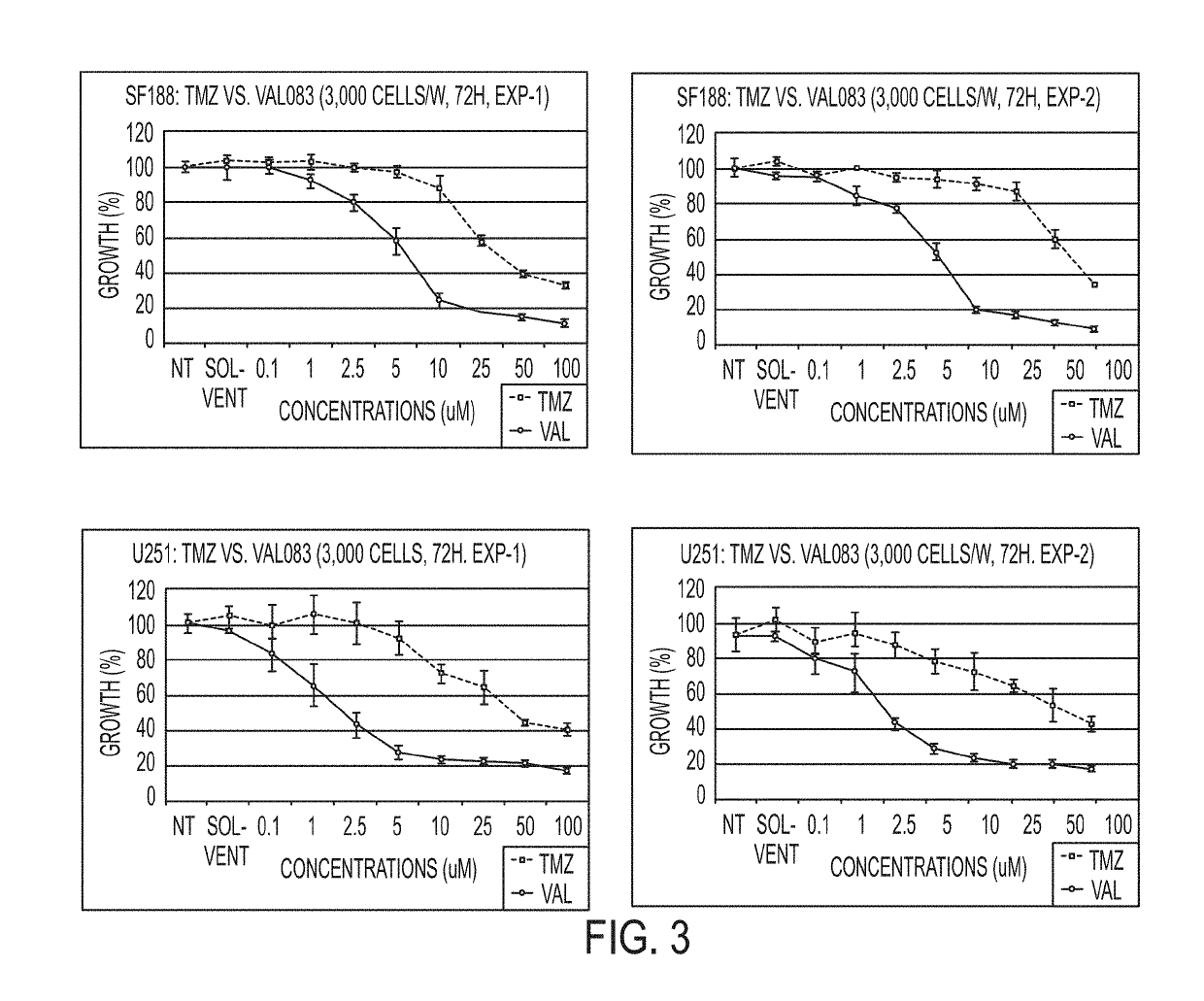
[1565]FIG. 3 shows the results for survival for SF188 (top panels), U251 (middle panels), and T98G (bottom panels) for 0.1, 1, 2.5, 5, 10, 25, 50, and 100 μM of temozolomide (TMZ) and dianhydrogalactitol (VAL). Two experiments are shown for each cell line.
[1566]FIG. 4 shows that dianhydrogalactitol at 5 μM provided more than 95% suppression of colony formation in...
example 3
Activity of Dianhydrogalactitol is Independent of MMR
[1600]The activity of dianhydrogalactitol is independent of MMR and independent of p53 status. The results of this Example are from studies with ovarian cancer cell lines; however, as MMR is a mechanism of DNA repair that is associated with the development of resistance to chemotherapy in central nervous system malignancies, including juvenile glioblastoma and medulloblastoma, the development of therapies that are independent of MMR is important for the use of such therapies in a manner that does not provoke drug resistance.
[1601]Methods:
[1602]The cell lines used were verified for tissue type by molecular analysis and were free of mycoplasma infection. The p53 status was established from sequencing the DNA. The drugs used were obtained from Sigma (cisplatin and oxaliplatin) or supplied by Del Mar Pharmaceuticals (dianhydrogalactitol).
[1603]The analysis of cytotoxicity, depicted as IC50 (concentration inhibiting growth of cells by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com