Implantable medical device with drug filled holes
a technology of implantable medical devices and holes, which is applied in the field of therapeutic agent delivery devices, can solve the problems of increasing trauma and risk to patients, restenosis is a major complication, and the surface coating can provide little actual control over the release kinetics of beneficial agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation Comprising a Therapeutic Agent within the Protective Layer
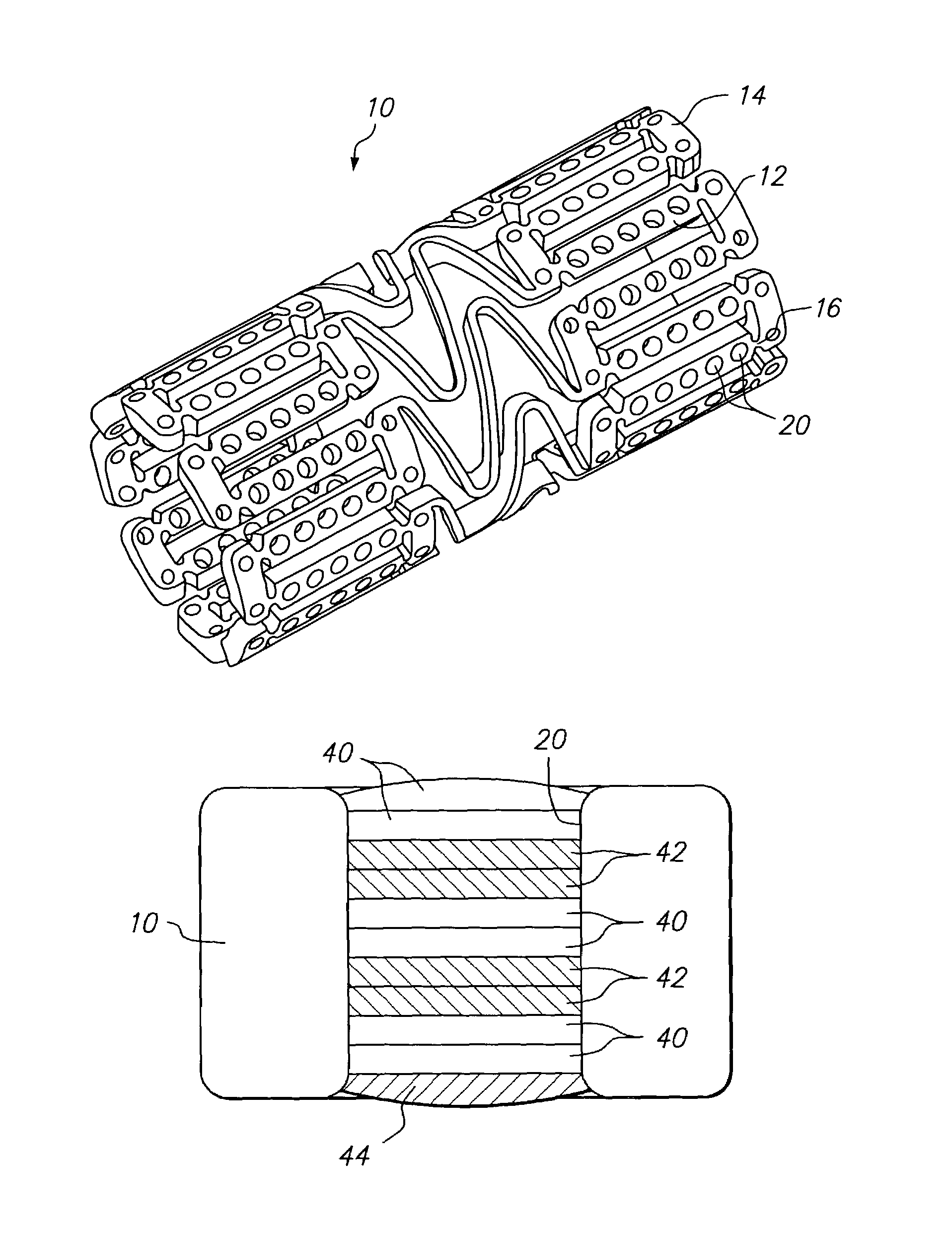
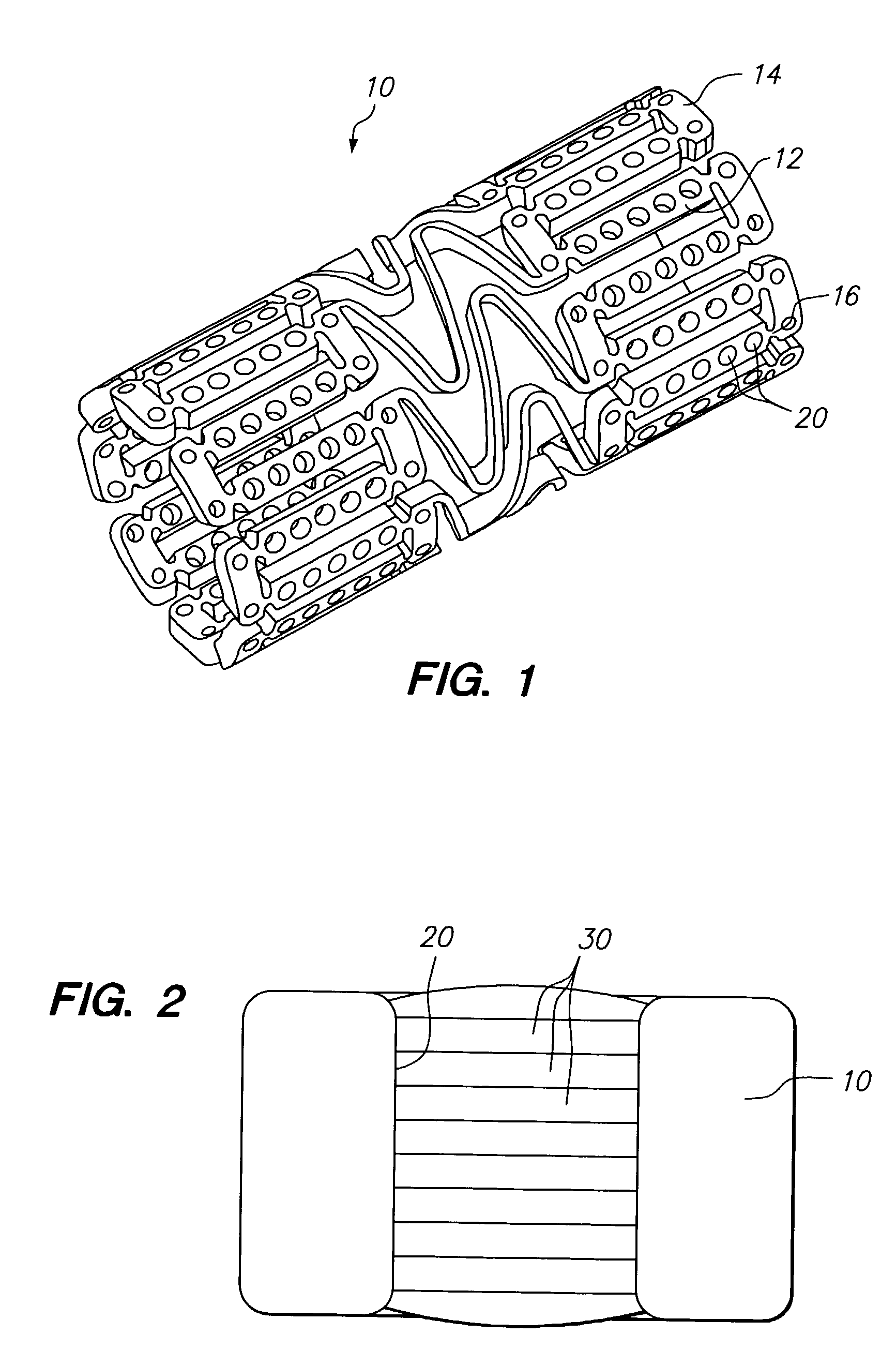
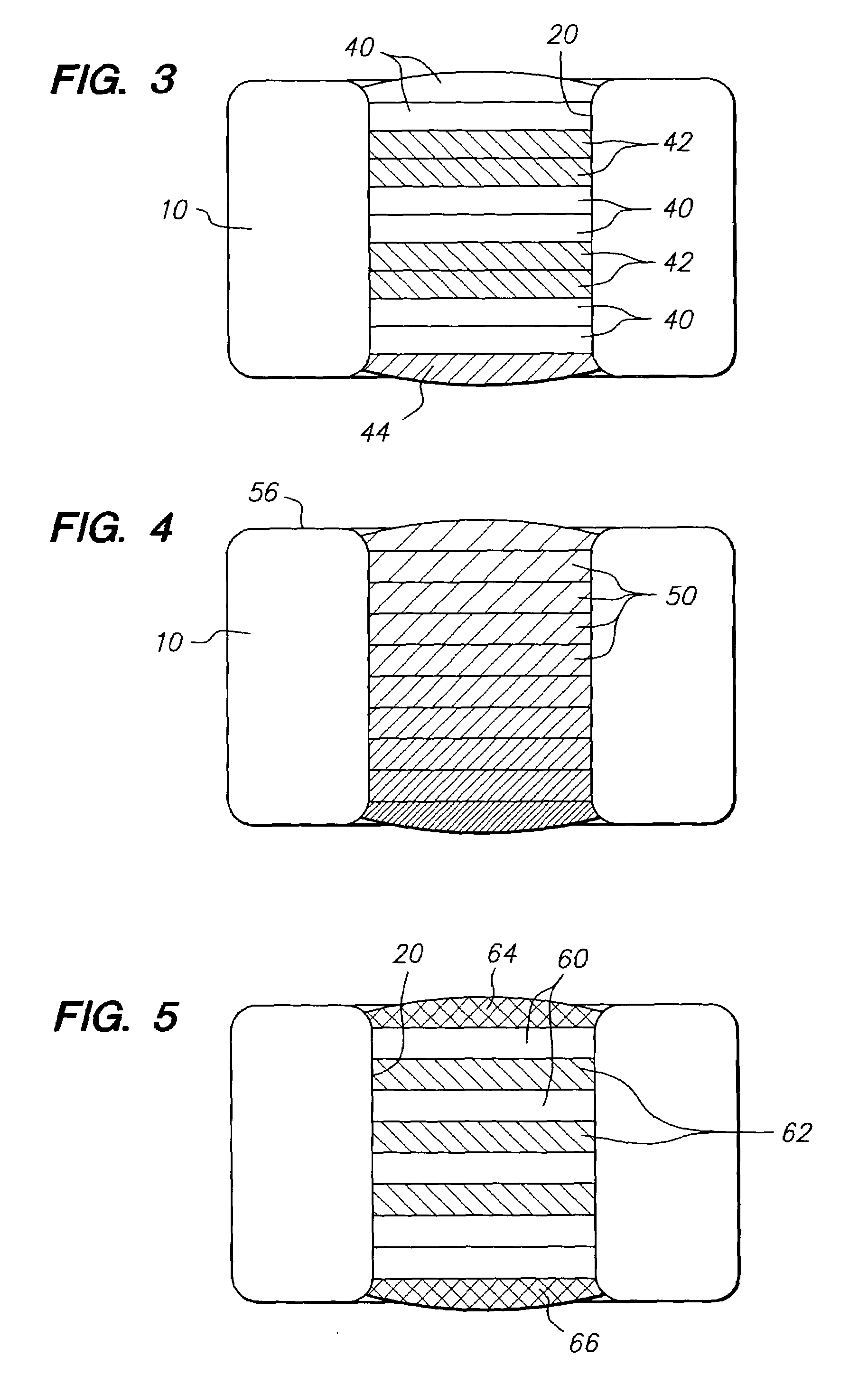
[0100]A first mixture of poly(lactide-co-glycolide) (PLGA) (Birmingham Polymers, Inc), lactide:glycolide: 85:15, (Mv>100,000 Daltons) 7% wt. and a suitable organic solvent, such as DMSO, NMP, or DMAC 93% wt. is prepared. The mixture is loaded dropwise into holes in the stent, then the solvent is evaporated to begin formation of the barrier layer. A second barrier layer is laid over the first by the same method of filling polymer solution into the hole followed by solvent evaporation. The process is continued until five individual layers have been laid down to form the barrier layer.
[0101]A second mixture of a limus, such as sirolimus, 3% solids basis, and dipalmitoyl phosphatidylcholine (DPPC), 7% solids basis, in a suitable organic solvent, such as DMSO, is introduced into holes in the stent over the barrier layer. The solvent is evaporated to form a drug filled protective layer and the filling and evaporation pr...
example 2
Formulation Comprising Therapeutic Agents in Therapeutic Agent Layers and a Protective Layer Separating the Therapeutic Agent Layers
[0104]A first mixture of poly(lactide-co-glycolide) (PLGA), lactide:glycolide: 85:15, (Mv>100,000 Daltons) 7% wt. and a suitable organic solvent, such as DMSO, 93% wt. is prepared. The mixture is loaded drop-wise into holes in the stent, and the solvent is then evaporated to form the barrier layer. A second barrier layer is laid over the first by the same method of filling polymer solution into the hole followed by solvent evaporation. The process is continued until five individual layers have been laid down to form the barrier layer.
[0105]A second mixture of an PCN-1 ribozyme, 8% solids basis, and poly(vinylpyrrolidone) (PVP), molecular weight 8,000 daltons, 2% solids basis, in an mixed solvent of RNA-ase / DNA-ase free water, 50% vol., and dimethyl sulfoxide (DMSO), 50% vol., is introduced into holes in the stent over the barrier layer. The solvent is e...
example 3
Formulation Comprising a Therapeutic Agent in a Therapeutic Agent Layer and a Protective Layer Containing an Activating Agent
[0110]A first mixture of high molecular weight poly(lactide-co-glycolide) (PLGA), lactide:glycolide: 50:50 (Mv>100,000 Daltons), 7% wt. and a suitable organic solvent, such as DMSO, 93% wt. is prepared. The mixture is loaded drop-wise into holes in the stent, then the solvent is evaporated to form the barrier layer. A second barrier layer is laid over the first by the same method of filling polymer solution into the hole followed by solvent evaporation. The process is continued until five individual layers have been laid down to form the complete barrier layer.
[0111]A second mixture of chymotrypsin, 3% solids basis, and polyvinyl pyrrolidone, 7% solids basis, in a solvent mixture of water:DMSO: 50:50 is introduced into holes in the stent over the barrier layer. The solvent is evaporated to form an activating ester hydrolytic enzyme filled protective layer and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com