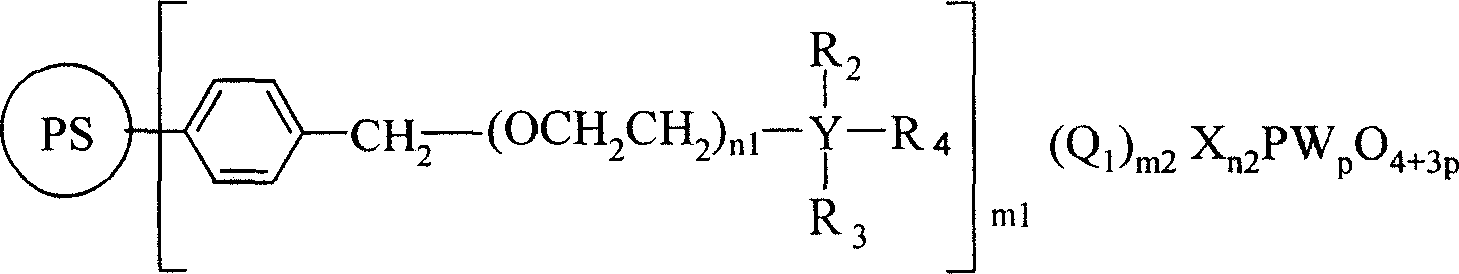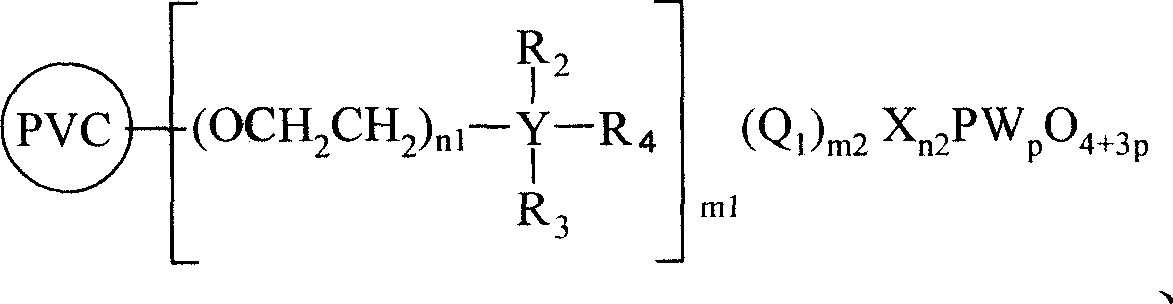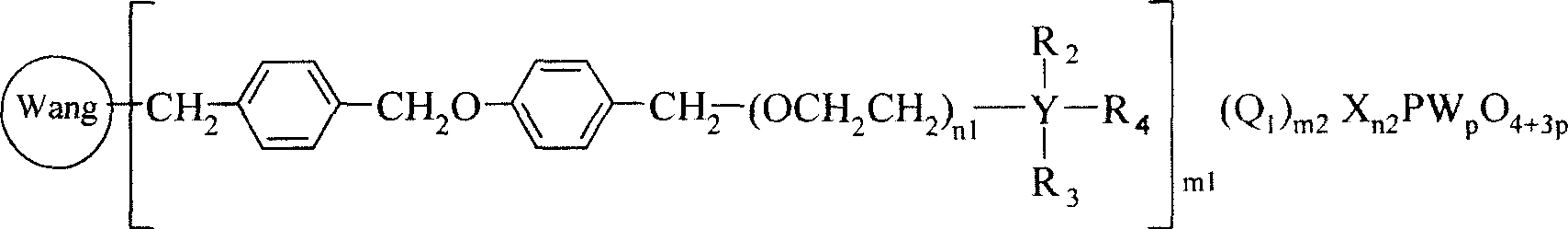No-solvent process of epoxidizing cyclohexene with hydrogen peroxide to prepare cyclohexane epoxide
A technology of epoxycyclohexane and hydrogen peroxide, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of activity or selectivity reduction and production capacity reduction , the process is difficult to industrialize and other issues, to achieve high selectivity and yield, reduce interfacial tension, simple reaction system effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1, cyclohexene 40mL, 35% hydrogen peroxide 20mL (molar ratio 1.75: 1), chloromethylated polystyrene (PS) supports N, N-dimethyl octadecyl phosphotungstic heteropoly acid (PS-CH 2 N(CH 3 ) 2 C 18 h 37 h 2 PW 4 o 16 )2g, NaHCO 3 0.05g, react at 50°C for 6h, filter out chloromethylated polystyrene (PS) supported by N,N-dimethyloctadecyl phosphotungstic heteropolyacid, separate the oil phase, and rectify to obtain epoxy Cyclohexane. Gas chromatographic analysis of the oil phase and determination of the hydrogen peroxide content in the water phase show that the conversion rate of hydrogen peroxide can reach 95.1%, the selectivity of epoxycyclohexane can reach 98.5%, and the yield of epoxycyclohexane can reach 93.3%. The infrared spectrum data of product epoxy cyclohexane is as follows (cm -1 ): 2936.8, 2936.8, 2672.9, 1458.5, 1437.9, 1356.9, 1260.0, 1187.4, 1168.3, 1086.6, 1036.6, 989.1, 966.2, 890.8, 877.0, 837.1, 780.9, 746.0.
[0037] Under the above-m...
Embodiment 2
[0038] Example 2, cyclohexene 40mL, 30% hydrogen peroxide 25mL (molar ratio 1.6:1), chloromethylated polystyrene supported polyethylene glycol N, N-dimethyl octadecyl ammonium phosphotungstic heteropoly Acid (PS-(OCH 2 CH 2 ) 15 N(CH 3 ) 2 C 18 h 37 NaHPW 4 o 16 )2.5g, CaCl 2 0.025g, react at 40°C for 8h, filter out chloromethylated polystyrene-supported polyethylene glycol N,N-dimethyloctadecylammonium phosphotungstic heteropolyacid, separate the oil phase, and rectify to obtain cyclo Oxycyclohexane. The infrared spectroscopic data of product epoxycyclohexane is as embodiment 1, and oil phase carries out gas chromatography analysis, and water phase measures hydrogen peroxide content as can be known, and the conversion rate of hydrogen peroxide reaches 97.4%, and the selectivity of epoxycyclohexane can reach 97.5%, The yield of epoxycyclohexane can reach 94.5%.
[0039] Under the above reaction conditions, catalyst A component --- chloromethylated polystyrene suppo...
Embodiment 3
[0040] Example 3, cyclohexene 40mL, 30% hydrogen peroxide 20mL (molar ratio 2: 1), chloromethylated polystyrene resin supported N, N-dimethyldodecamonium phosphotungstic heteropolyacid (PS-CH 2 N(CH 3 ) 2 C 12 h 25 h 2 PW 4 o 16 ) 0.2g, KCl 0.2g, react at 60°C for 4h, filter out chloromethylated polystyrene resin supported N,N-dimethyldodecamonium phosphotungstic heteropolyacid, separate the oil phase, and rectify to obtain ring Oxycyclohexane. The infrared spectroscopic data of product epoxycyclohexane is as embodiment 1, and oil phase carries out gas chromatography analysis, and water phase measures hydrogen peroxide content as can be known, and the conversion rate of hydrogen peroxide reaches 94.3%, and the selectivity of epoxycyclohexane can reach 95.4%, The yield of epoxycyclohexane can reach 90.0%.
[0041] Under the above reaction conditions, catalyst A component --- chloromethylated polystyrene resin supported N, N-dimethyldodecamonium phosphotungstic heteropol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com