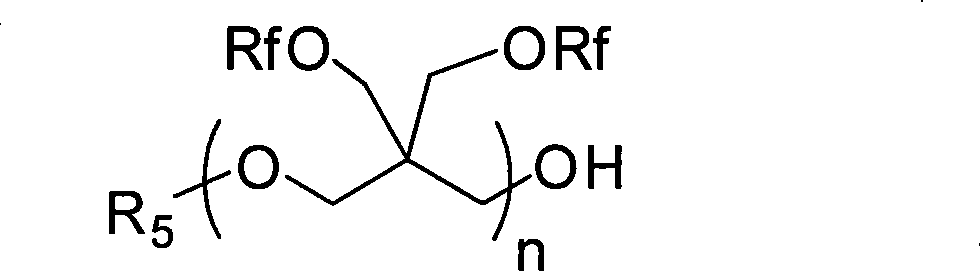
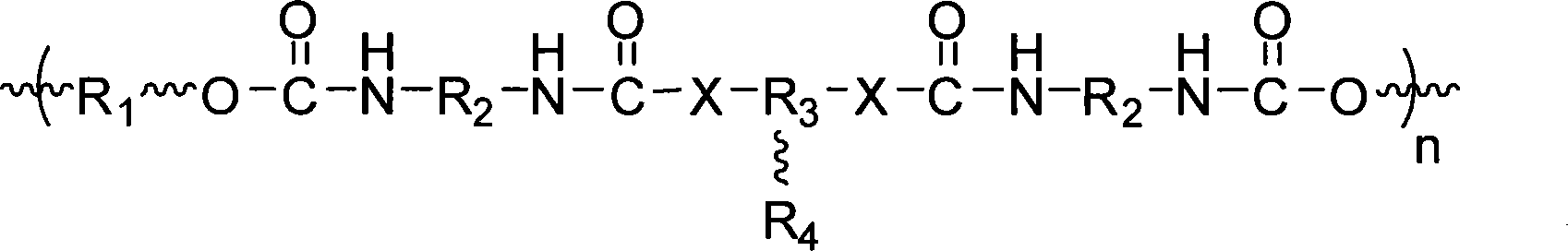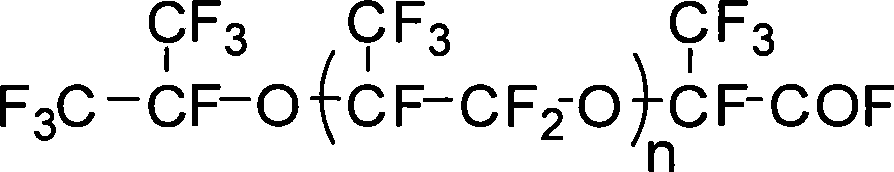Fluorine-containing polyether graft modified aqueous polyurethane and preparation and application thereof
A technology of water-based polyurethane and graft modification, which is applied in the field of water-based polyurethane, which can solve the problems of pollution and undeveloped problems, and achieve the effects of pollution-free waterproof, excellent self-cleaning performance, and good yellowing resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add tribromoneopentyl alcohol (20.0g, 61.5mmol), NaOH (10wt%, 75g), phase transfer catalyst tetrabutylammonium bromide TBAB (0.79 g, 2.46mmol) and CH 2 Cl 2 (100 mL), stirred evenly by magnetic force, then raised the temperature to 35°C for 12 hours, stopped the reaction, and cooled to room temperature. The layers were allowed to stand, the organic matter in the lower layer was removed, the aqueous layer was extracted with dichloromethane, the organic layers were combined, and dried over anhydrous magnesium sulfate. The organic solvent was removed under vacuum. Vacuum distillation, collecting 92-94oC / 4mmHg fraction. 3,3-Dibromomethylbutene oxide was obtained as a colorless liquid (10.5 g, 70% yield). 1H NMR (300 MHz, CDCl3) δ: 3.86 (s, 4H), 4.44 (s, 4H).
[0046] Under nitrogen protection, 3,3-dibromomethylbutene oxide (10.0 g, 40.9 mmol) was added into a 100 mL three-neck flask equipped with a reflux condenser, dropping funnel and thermometer. The phase transfer ...
Embodiment 2
[0053] Under nitrogen protection, neopentyl glycol (7.37g, 70.9mmol), BF 3 ·OEt 2 (4.03g, 28.4mmol) and anhydrous dichloromethane (185mL) were added to a dry 500mL three-necked flask. Stir at room temperature for 30 minutes. Fluorine-containing butylene oxide (120 g, 0.426 mol) was slowly added dropwise to the reaction mixture. This reaction was exothermic, and a slight rise in temperature was found at this time. After the addition was complete, the reaction was continued at room temperature for 6 hours. stop the reaction, the residual BF 3 ·OEt 2 It can be washed off with 2.5wt% sodium bicarbonate aqueous solution, and then washed with hot water at 40°C, and finally the solvent is removed under vacuum. The resulting colorless viscous dihydroxyl fluorine-containing polybutylene oxide with hydroxyl groups at the end of the obtained polymer has a yield of 95% and a hydroxyl value of 42.15-39.14 mgKOH / g.
Embodiment 3
[0055] Under nitrogen protection, methanol (2.27g, 70.9mmol), BF 3 ·OEt 2 (4.03g, 28.4mmol) and anhydrous dichloromethane (185mL) were added to a dry 500mL three-necked flask. Stir at room temperature for 30 minutes. Fluorine-containing butylene oxide (120 g, 0.426 mol) was slowly added dropwise to the reaction mixture. This reaction was exothermic, and a slight rise in temperature was found at this time. After the addition was complete, the reaction was continued at room temperature for 6 hours. Terminate the reaction with 50 ml of 5% methanol solution of sodium methoxide. After stirring for 1 hour, wash with 2.5 wt % sodium bicarbonate aqueous solution and hot water at 40° C., and finally remove the solvent under vacuum. The resulting polymer is a colorless and viscous waxy substance with a methoxy group at one end and a hydroxyl group at the other end, that is, monohydroxyl fluorine-containing polybutylene oxide, with a yield of 93% and a hydroxyl value of 25.3 mgKOH / g. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com