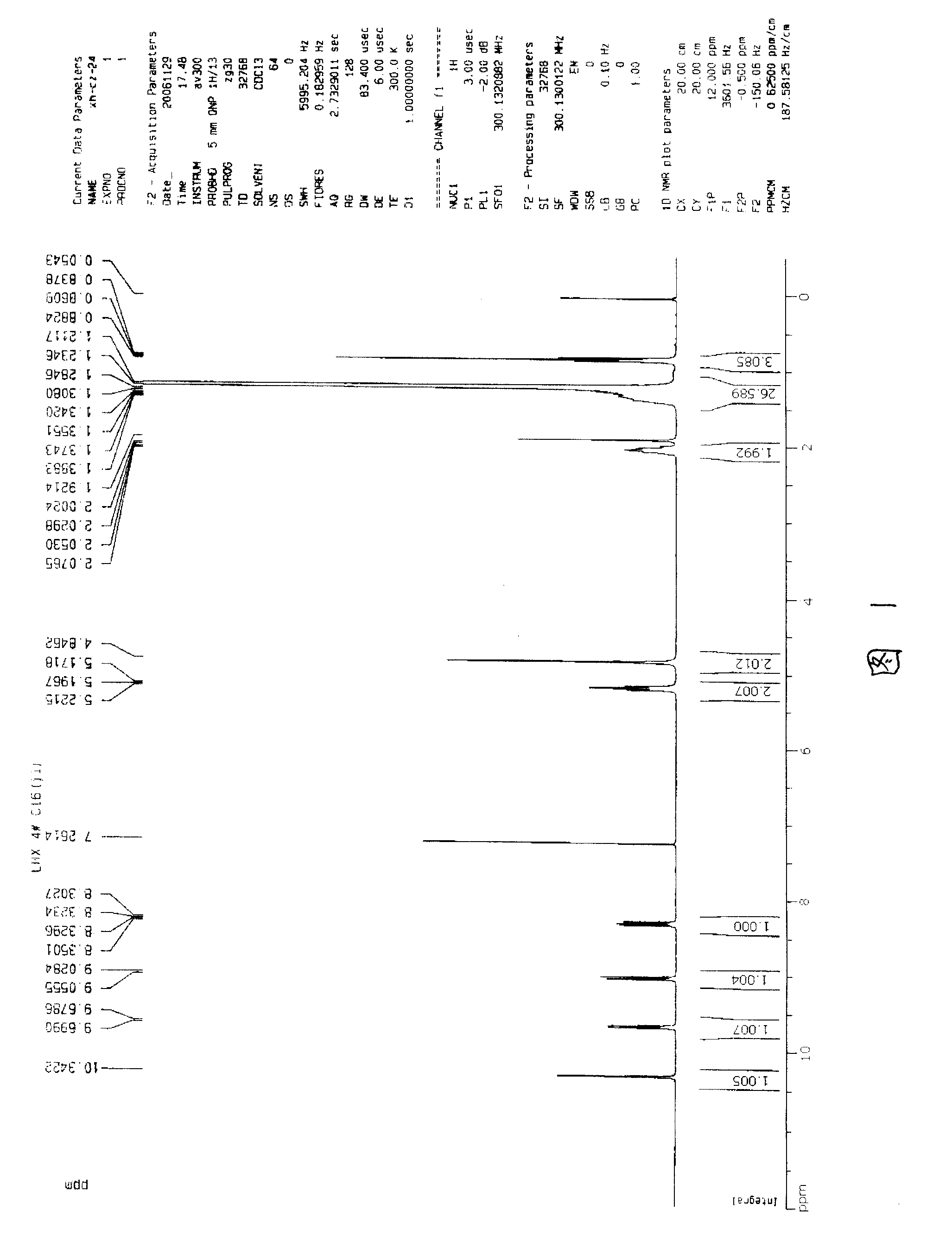Gemini nicotinate quaternary ammonium salt and method for making same
A quaternary ammonium salt and nicotinic acid technology, applied in chemical instruments and methods, organic chemistry, transportation and packaging, etc., can solve the problems of decreased use effect, low bactericidal concentration, high water solubility, etc., and achieves weak foaming, high The effect of surface activity and strong sustained release ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of brominated di-N-alkylnicotinic acid ethylene glycol / diethylene glycol / triethylene glycol ester (EQn-m-n)
[0034] Synthesis of EQ16-2-16
[0035] Add nicotinic acid and thionyl chloride at a molar ratio of 1:5 into the reaction flask, stir and reflux, and NaOH solution absorbs the tail gas. After reacting for 4 hours, it was cooled, and the excess thionyl chloride was spin-dried under reduced pressure to obtain white needle-like crystals. Using dry chloroform as a solvent, slowly drop triethylene glycol with a molar ratio of 1:2 to nicotinic acid, NaOH solution to absorb tail gas, and reflux for 10 h. After cooling, the solvent was spin-dried, and an appropriate amount of water was added to dissolve it, and saturated Na 2 CO 3 Adjust the pH of the aqueous solution to be weakly alkaline, separate the organic phase, extract the aqueous phase with ethyl acetate 2-3 times, combine the organic phases, spin dry and pass through the column with ethyl acetate,...
Embodiment 2
[0044] Structural Characterization of Brominated Di-N-Alkyl Nicotinic Acid Ethylene Glycol / Diethylene Glycol / Triethylene Glycol Ester (EQn-m-n)
[0045] Structural characterization of EQ16-0-16
[0046]
[0047] Referring to Fig. 1, Fig. 1 is the hydrogen spectrogram of EQ16-0-16, according to the spectrogram the chemical shift and related parameters of EQ16-0-16 are listed in the following table 1:
[0048] Table 1EQ16-0-16 1 HNMR parameters
[0049] absorption peak
δ(ppm)
number of small peaks
ratio of hydrogen atoms
1
2
3
4
5
6
7
8
9
10.34
9.70
9.05
8.35
5.22
4.84
2.07
1.21-1.1.38
0.88
S
D.
D.
Q
T
S
m
m
T
1.005
1.007
1.004
1.000
2.007
2.012
1.992
26.598
3.085
1
1
1
1
...
Embodiment 3
[0067] Study on Surface Activity of Brominated Di-N-Alkyl Nicotinic Acid Ethylene Glycol / Diethylene Glycol / Triethylene Glycol Ester (EQn-m-n)
[0068] The surfactant to be measured is prepared into a series of aqueous solutions of a certain concentration with twice distilled water, and the surface tension of the aqueous surfactant solution is measured by the suspension ring method at 20°C. The measurement results are shown in Figure 4 and Table 4.
[0069] It can be seen from Figure 4 that with the addition of this series of surfactants, the surface tension of water drops sharply, and the concentration increases, the surface tension decreases slowly, and finally tends to be stable. The alkyl chain grows and the surface activity increases.
[0070] Table 4 cmc, γ of several surfactants cmc and C 20
[0071] substances
cmc (mmol / l)
gamma cmc (mN / m)
C 20 (mmol / l)
EQ08-0-08
3.65
50.5
3.022
[0072] EQ10-0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com