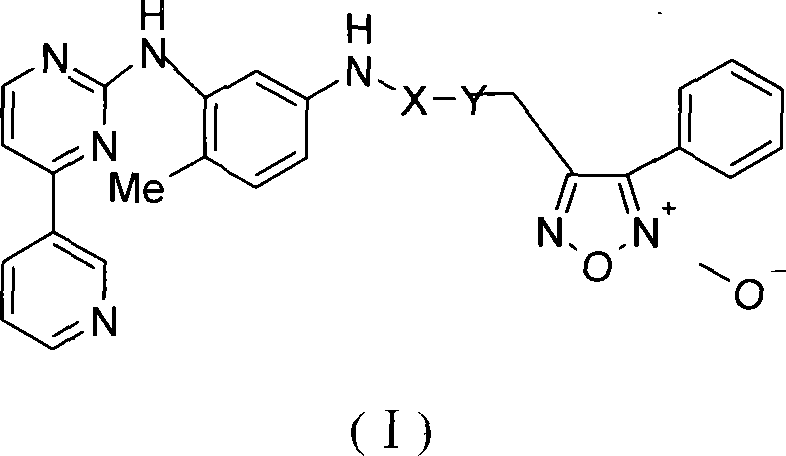
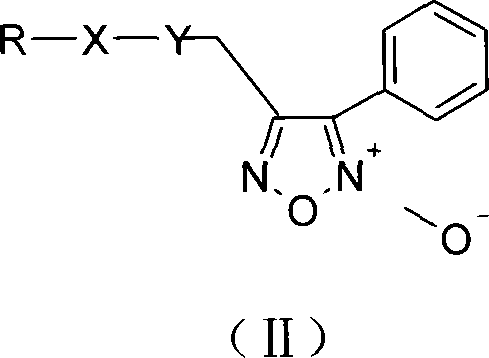
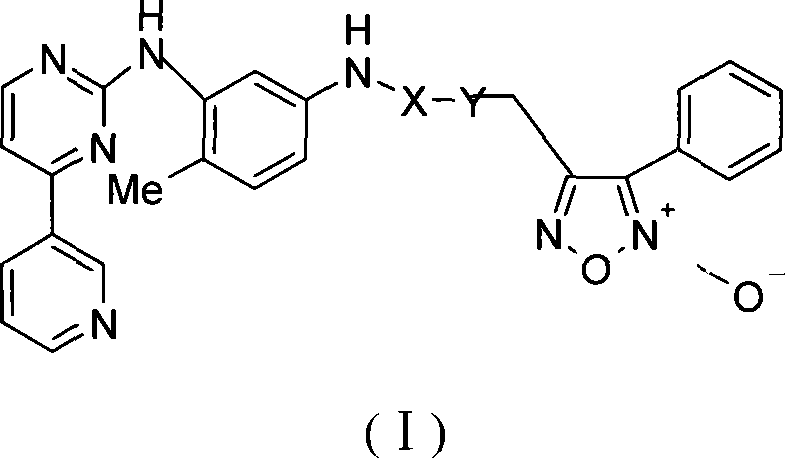
Phenyl urazan nitrogen nitric oxide donor 2-aniline pyrimidine derivatives, preparation method, compound containing the same and use thereof
A compound and oxide technology, applied in the field of novel phenylfurazan nitrogen monoxide donor 2-aniline pyrimidine derivatives, can solve the problems of poor controllability, short half-life, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidinamine (A) and compound intermediate of general formula (II)
[0072] Preparation of N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidinamine (A)
[0073] Add N,N-dimethylformamide dimethyl acetal (N,N-dimethylformamide dimethyl acetal) (156.5g, 1.27mol) to 3-acetylpyridine (100g, 0.826mol) and reflux for 23 hours, then cool to 0°C , add the mixed solution diethyl ether and n-hexane (3:2, v / v) (500ml), stir for 4 hours, filter the obtained solid and rinse with diethyl ether and n-hexane (3:2, v / v) (400ml), dry to obtain 3-Dimethylamino-1-(3-pyridyl)-2-propen-1-one;
[0074] 2-Methyl-5-nitroaniline (100g, 0.657mol) was dissolved in 250ml of absolute ethanol, at 30-40°C, 65% nitric acid (54.5ml, 0.788mol) was added dropwise within 1 hour, when the exothermic When the reaction stopped, cyanamide: water (42g: 42g) was added thereto, the brown solution was refluxed for 24 hours, cooled to 0°C, filtered, eth...
Embodiment 2
[0100] 2-N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]-phenyl]acetic acid-4-phenyl-1,2,5-oxadiazole-2 -Oxide-3-methyl ester
[0101] 3-Hydroxymethyl-4-phenyl-1,2,5-oxadiazole-2-oxide (2) (2.4g, 12.5mmol) was dissolved in 40ml of anhydrous dichloromethane, and bromoacetic acid ( 1.91g, 13.74mmol), DCC (3.86g, 18.74mmol) and a catalytic amount of DMAP were added in batches under an ice bath and stirred to dissolve, stirred at room temperature for three hours, filtered, washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, and concentrated to obtain Light yellow solid 3-bromoacetic acid (-4-phenyl-1,2,5-oxadiazole-2-oxide) methyl ester 3.58g. Yield 91.8%. N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidinamine (2.22g, 8.01mmol) and triethylamine (1.45ml, 10.41mmol) were dissolved in 50ml of chloroform , after stirring to dissolve, add 3-bromoacetic acid (-4-phenyl-1,2,5-oxadiazole-2-oxide) methyl ester 3.01g, 9.62mmol) and polyethylene g...
Embodiment 3
[0107] 3-N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]-phenyl]methyl-4-phenyl-1,2,5-oxadiazole- Synthesis of 2-oxides
[0108] N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidinamine (A) (2.77g, 10mmol) was dissolved in anhydrous potassium carbonate (1.79g, 13mmol) In 60ml tetrahydrofuran, after stirring for 20 minutes, add 3-chloromethyl-4-yl-1,2,5-oxadiazole-2-oxide (3) (2.10g, 10mmol) and a catalytic amount of tetrabutyl Ammonium bromide (TBAB) was heated to reflux for 4 hours. Filtration, the filtrate was concentrated, the residue was dissolved in ethyl acetate, washed with 10% aqueous sodium bicarbonate, dried over anhydrous sodium sulfate, filtered, column chromatography (ethyl acetate:petroleum ether=5:1), ethyl acetate Crystallization gave 3-N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]-phenyl]methyl-4-phenyl-1,2,5-oxadi Azole-2-oxide 1.35 g. Yield 74.83%, mp: 154.9-157.0°C, ESI-MS m / z: 451, 474 [M+Na] + ;
[0109] IR (KBr, v (cm -1 )) 3257(N-H), 302...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com