Process for preparing acephate from ethenone
A technology of acephate raw powder and ketene, which is applied in the field of pesticide acephate preparation and high-purity acephate preparation from ketene, which can solve the problem of lower product yield and purity, low total separation yield, Separation difficulties and other problems, to reduce decomposition loss, overcome the neutralization process, reduce the effect of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
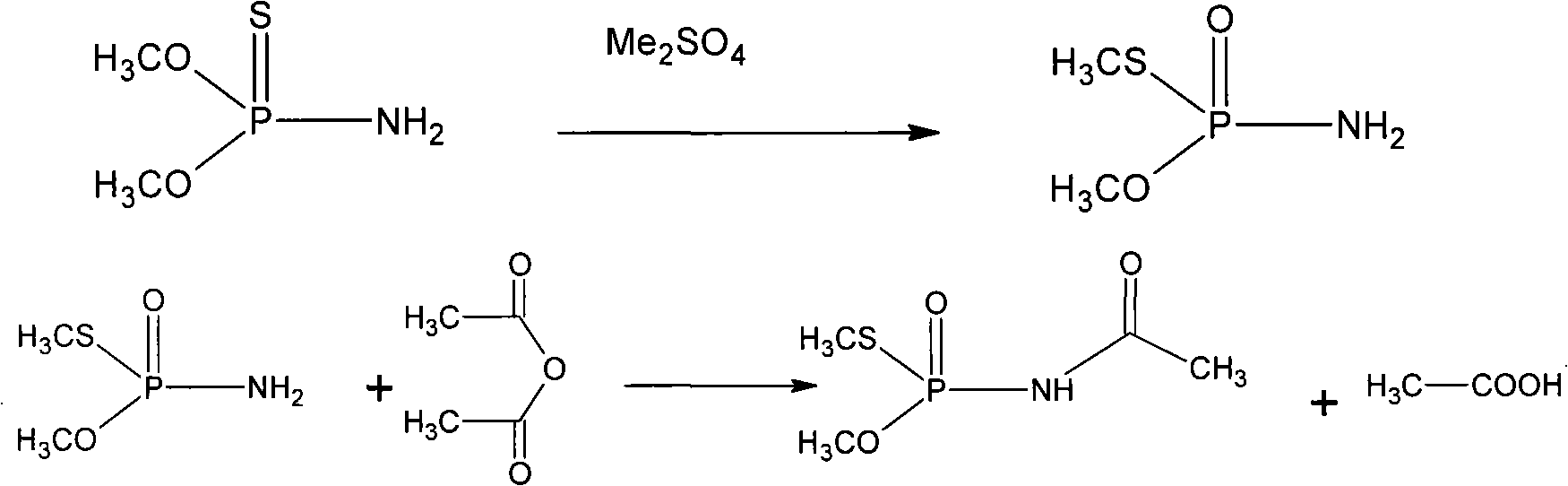
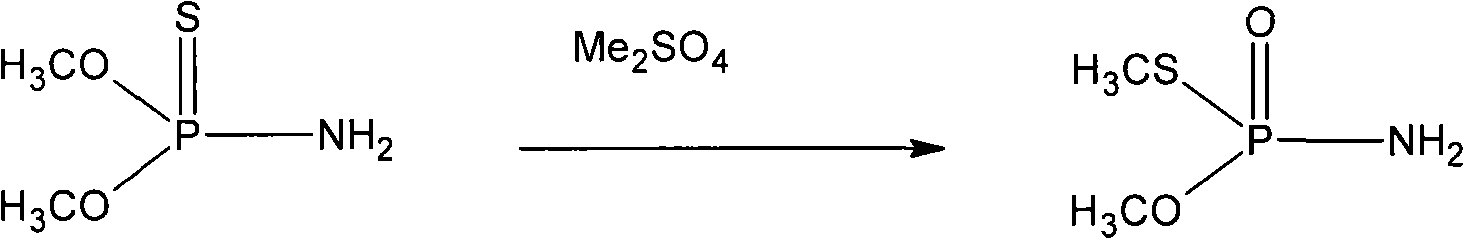
[0033] In a 1000mL four-necked flask with a stirrer, condenser, thermometer and vent tube, add 472g of O, O-dimethylphosphorothioate, add 28g of dimethyl sulfate, carry out isomerization reaction to obtain 73.5% O,S-Dimethylphosphorothioate 500g. The reaction temperature is 50-65° C., and the reaction time is 3 hours.
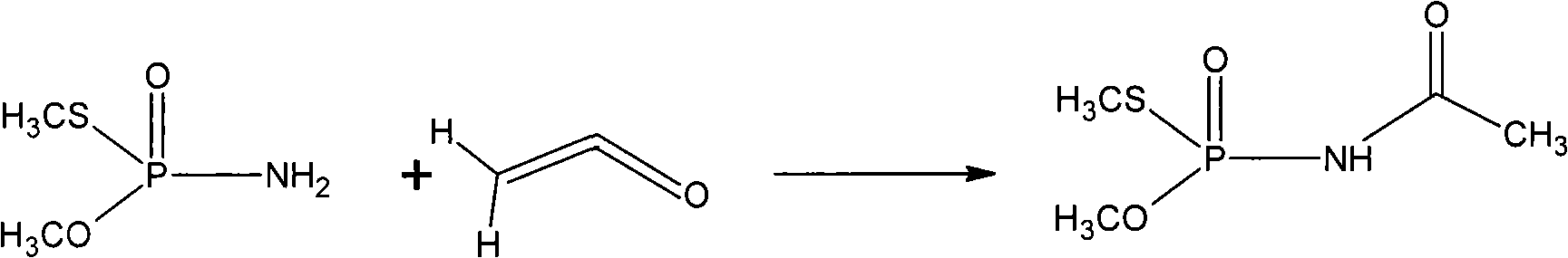
[0034] Add 300mL of dichloroethane and 1.8g of p-toluenesulfonic acid to the above reaction product, under vigorous stirring, control the temperature at 20°C with ice-salt water, and feed the newly produced mixed gas of ketene and nitrogen to control about 40-50g Feed ketene at a rate of / hr, and stop the reaction when the reaction mixture no longer absorbs ketene. The reaction time is 4-6 hours. Cool down to 0-5°C, a large amount of solids are precipitated, filter, the mother liquor can be recycled to the next batch of reaction solution, and the product is washed with 100mL dichloroethane, and vacuum-dried to obtain 430g of acephate. The yield is 90.7% (with...
Embodiment 2
[0036] O, the preparation of S-dimethylphosphorothioate is the same as in Example 1.
[0037] In a 1000mL four-neck flask with a stirrer, condenser, thermometer and vent tube, add 500g of O, S-dimethylphosphorothioate (methamidophos), 300mL of dichloromethane and 1.5g of boron trifluoride - Diethyl ether, under vigorous stirring, control the temperature in a water bath to 20°C, feed the newly produced mixed gas of ketene and nitrogen, control the speed of about 40-50g / hr to feed ketene, when the reaction mixture no longer absorbs ketene stop responding. The reaction time was 6 hours. Cool down to 0-5°C, a large amount of solids are precipitated, filter, the mother liquor can be recycled to the next batch of reaction liquid, wash the product with 100mL dichloroethane, and vacuum dry to obtain 420g of acephate, the yield is 88.7%, and the product is white Solid purity 98.5%.
Embodiment 3
[0039] O, the preparation of S-dimethylphosphorothioate is the same as in Example 1.
[0040] In a 1000mL four-necked flask equipped with a stirrer, condenser, thermometer and vent tube, add 500g of O, S-dimethylphosphorothioate (methamidophos), 50mL of acetonitrile, 250mL of dichloromethane, 1.5g of tris Boron fluoride-ether and 2g dimethyl sulfate, under vigorous stirring, the temperature is controlled by a water bath at 25°C, and the newly produced mixed gas of ketene and nitrogen is introduced, and the rate of about 40-50g / hr is controlled to feed ketene. The reaction was stopped when the reaction mixture no longer took up ketene. The reaction time was 6 hours. Cool down to 0-5°C, a large amount of solids are precipitated, filter, the mother liquor can be recycled to the next batch of reaction liquid, wash the product with 100mL of dichloromethane, and dry in vacuum to obtain 425g of acephate, the yield is 89.7%, and the product is a white solid 98.5% purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com