Process for extracting copper by wet method from copper-containing sulfidization ore
A technology of sulfide ore and wet method, which is applied to the improvement of process efficiency, photographic technology, instruments, etc., can solve the problems of poor process adaptability, environmental pollution, difficult to handle sulfuric acid, etc., and achieve a wide range of raw materials, easy process operation, copper The effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
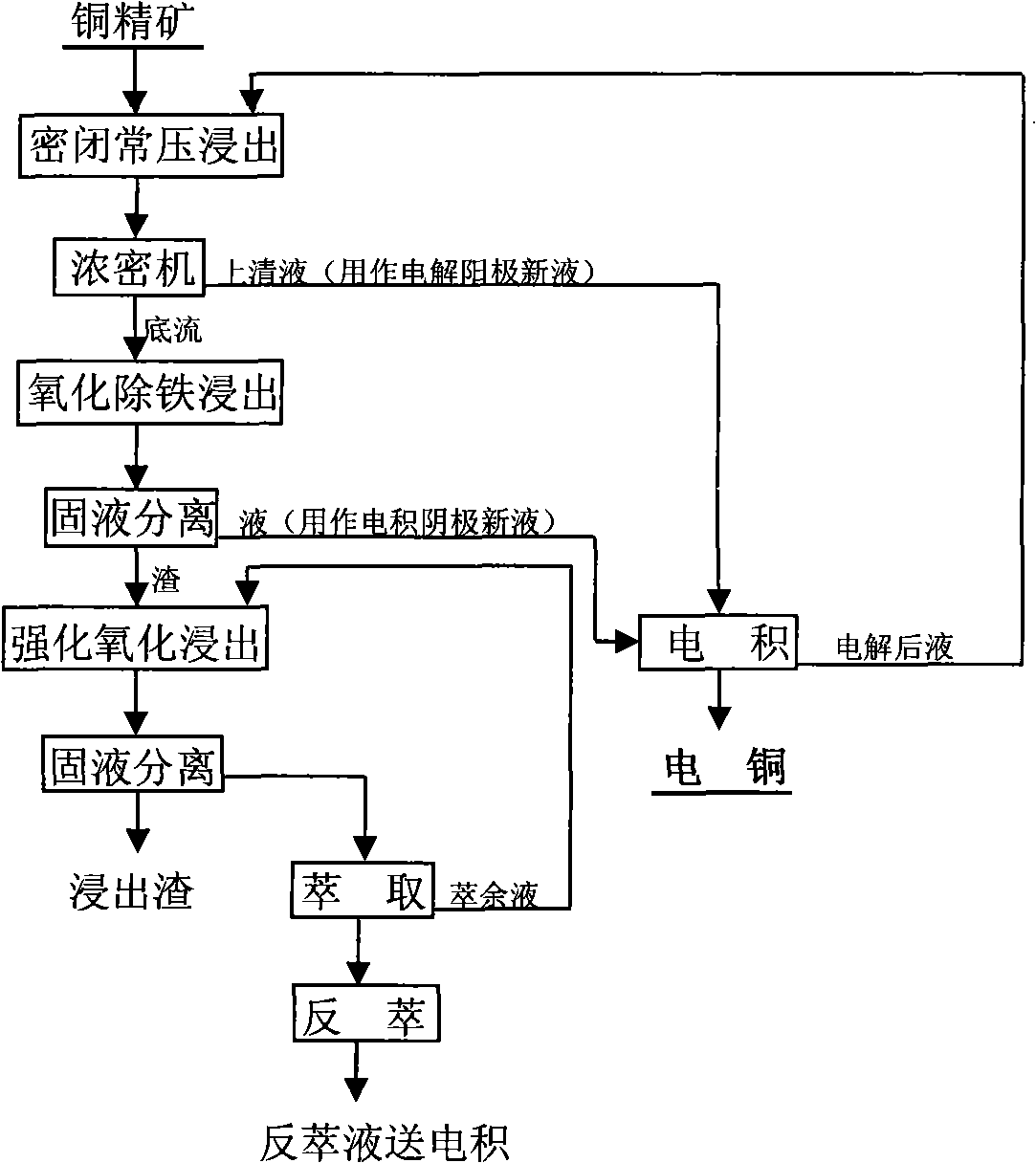
[0055] Add 100g of finely ground 1# copper concentrate to the GSA type 2L vertical titanium-lined autoclave, the concentrate contains copper: 14.17%, iron: 31.63%, sulfur: 32.19%, 600ml leachate, the main component of the leachate: free H 2 SO 4 : 60g / L, Cu 2+ : 15g / L, Fe 3+ 35g / L, heated to 110°C, leaching time 4h, after the reaction, the slurry is thick, and the thick overflow is sent to the anode chamber of the electrolytic cell for oxidation, and the thick underflow enters the next stage of oxidation and iron removal, and the underflow concentration is 33%. The leaching rate of Cu in the closed atmospheric pressure leaching section is 42%, and the leaching rate of iron is 25%. The leach solution contains Cu30g / L and Fe40g / L.
[0056] The dense underflow of the first stage of closed atmospheric pressure leaching and the electrowinning catholyte enter the second stage of oxygen pressure iron removal and leaching. The reaction is carried out in a GSA type 2L vertical titan...
Embodiment 2
[0060] One-stage closed atmospheric pressure leaching test conditions: leaching temperature 130°C, leaching time 6h, leaching liquid-solid mass ratio 4:1, main components of leaching liquid: free H 2 SO 4 : 250g / L, Cu 2+ : 15g / L, Fe 3+ 35g / L, others are the same as Example 1, the Cu leaching rate of the closed atmospheric pressure leaching section is 45%, and the iron leaching rate is 36%. The leach solution contains Cu40g / L and Fe50g / L.
[0061] Two-stage oxygen pressure iron removal leaching test conditions: leaching temperature: 170°C, leaching time 4h, air is introduced during the reaction process, others are the same as Example 1, Cu leaching rate in oxygen pressure iron removal leaching section is 25%, total Cu leaching rate is 70% , the iron in the leaching solution enters the slag, and the Fe in the solution is <2g / L.
[0062] Three-stage enhanced oxidation leaching test conditions: leaching temperature 130°C, leaching liquid-solid mass ratio 4:1, Cu 0.5g / L in lea...
Embodiment 3
[0064] One-stage closed atmospheric pressure leaching test conditions: leaching temperature 80°C, leaching time 4h, leaching liquid-solid mass ratio 6:1, main components of leaching liquid: free H 2 SO 4 : 180g / L, Cu 2+ : 15g / L, Fe 3+ 35g / L, others are the same as Example 1, the Cu leaching rate of the closed atmospheric pressure leaching section is 40%, and the iron leaching rate is 25%.
[0065] Two-stage oxygen pressure iron removal leaching test conditions: leaching temperature: 80°C, leaching time 3h, hydrogen peroxide was introduced in the reaction process, the others were the same as in example 1, Cu leaching rate in the oxygen pressure iron removal leaching section was 25%, and the total leaching rate of Cu was 70%. , the iron in the leaching solution enters the slag in the form of goethite, and the Fe in the solution is <2g / L.
[0066] Three-stage enhanced oxidation leaching test conditions: leaching temperature 110°C, leaching liquid-solid mass ratio 6:1, Cu 0.5g / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com