Soluble electro-green light organic molecule glass material and preparation method and use thereof
A technology of organic molecules and glass materials, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of high-resolution color display devices, achieve high film shape stability, convenient purification, and simple synthesis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the preparation of 2-(4-isobutoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane:
[0040]
[0041] 4-isobutoxy-1-bromobenzene (4.5g, 19.65mmol) was dissolved in dried tetrahydrofuran (40mL), and n-butyllithium (2.5M, 7.86mL, 19.65mL) was added dropwise at -78°C mmol), in N 2 React under atmosphere for 1 hour, then add 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborane (4.61mL, 19.65mmol) rapidly, and then gradually increase Reaction at room temperature for 8 hours. The reaction mixture was poured into water, and extracted with dichloromethane. The organic layer was washed with brine and washed with anhydrous MgSO 4 dry. After the solvent was removed under reduced pressure, it was separated with a silica gel column, and the eluent was petroleum ether to obtain a colorless transparent liquid.
Embodiment 2
[0042] Embodiment 2, the preparation of 4-bromo-7-(4-isobutoxyphenyl)-2,1,3-benzothiadiazole:
[0043]
[0044] 2-(4-isobutoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane (3.1g, 11.2mmol), 4,7-di Bromo-2,1,3-benzothiadiazole (4.26g, 14.6mmol), toluene (70mL), ethanol (25mL), and 2M aqueous sodium carbonate solution (20mL) were added to a two-necked flask, and nitrogen gas was bubbled to exhaust for 30 minute. The catalyst tetrakis(triphenylphosphine)palladium (195mg, 0.168mmol) was quickly added into the reaction flask, and then heated to 70°C for reflux reaction for 36 hours. After the mixture was cooled, it was washed with distilled water and extracted three times with toluene and dichloromethane respectively, and the organic phase was extracted with MgSO 4dry. A yellow-green solid was obtained by column chromatography.
Embodiment 3
[0045] The preparation of embodiment 3,3,5-two (4-isobutoxyphenyl) bromobenzene:
[0046]
[0047] 2-(4-isobutoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (5.58g, 20.2mmol), s-tribromobenzene ( 3.03g, 9.63mmol), toluene (50mL), ethanol (20mL), and 2M sodium carbonate aqueous solution (20mL) were added into a two-necked flask, and nitrogen gas was bubbled to exhaust for 30 minutes. The catalyst tetrakis(triphenylphosphine)palladium (0.69 g, 0.6 mmol) was quickly added into the reaction flask, and then heated to 90° C. for reflux reaction for 8 hours. After the mixture was cooled, it was washed with distilled water and extracted 3 times with dichloromethane, and the organic phase was washed with MgSO 4 After drying, a white solid was obtained by column chromatography. Example 4, 3,5-bis(4-isobutoxyphenyl)-1-(4,4,5,5-tetramethyl-1,3,2-dioxaboryl)benzene preparation:
PUM
| Property | Measurement | Unit |
|---|---|---|
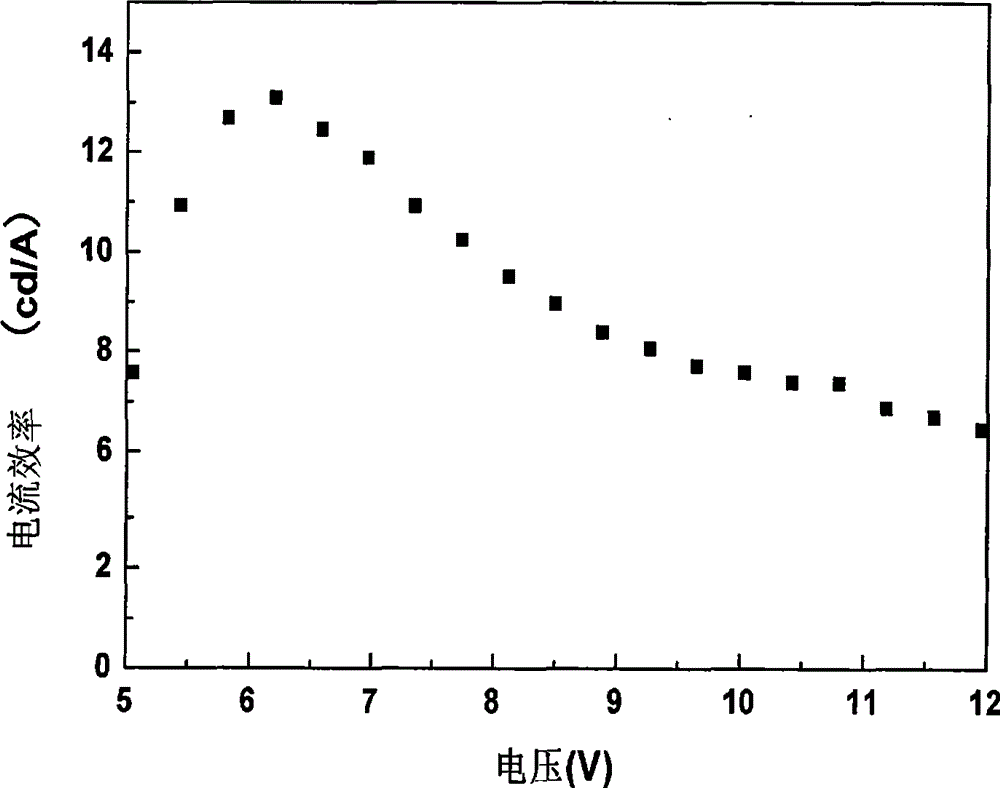
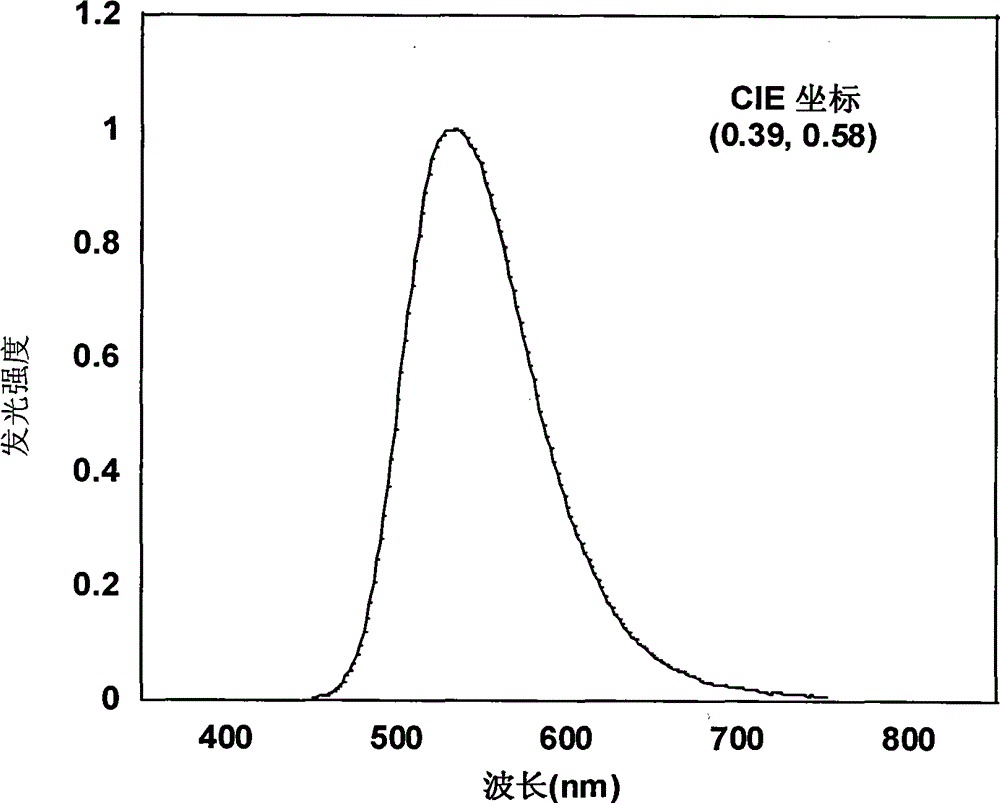
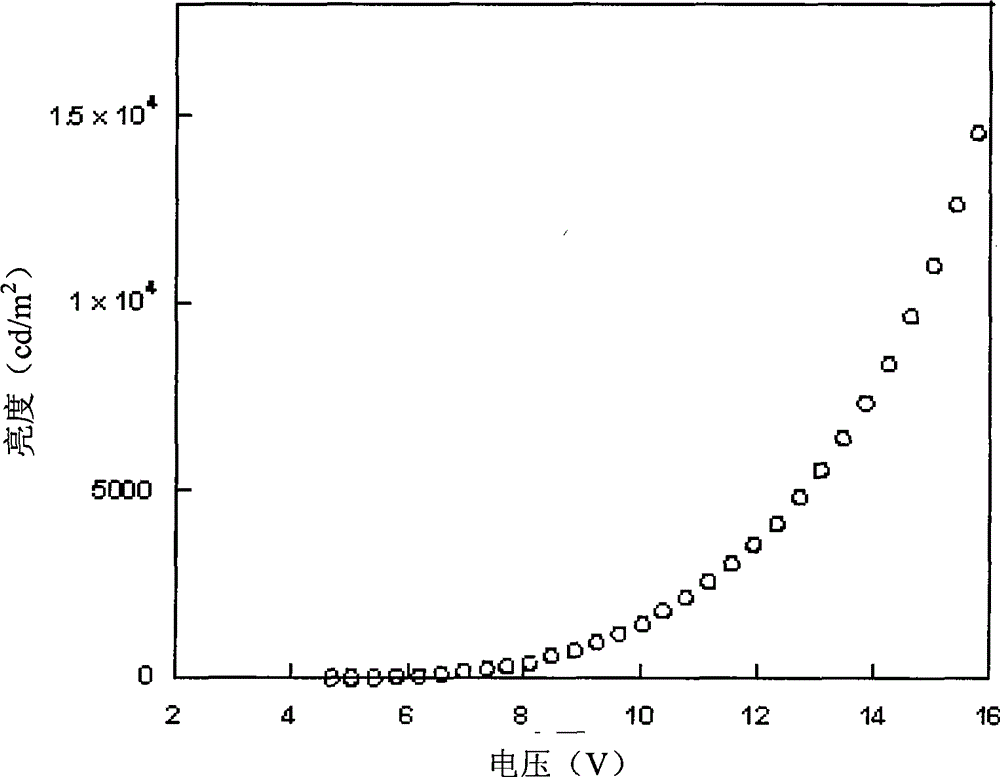
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com