5-fluorouracil-sn2-phosphatidyl choline copolymer as well as preparation method and application thereof
A technology of phosphatidylcholine and fluorouracil, applied in the field of medicine, can solve the problems such as the preparation method and use of 5-fluorouracil-phosphatidylcholine copolymer, the application of drug-phosphatidylcholine copolymer, side effects and the like have not been found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0201] Example: Take lysophosphatidylcholine containing palmitic acid residues as an example.
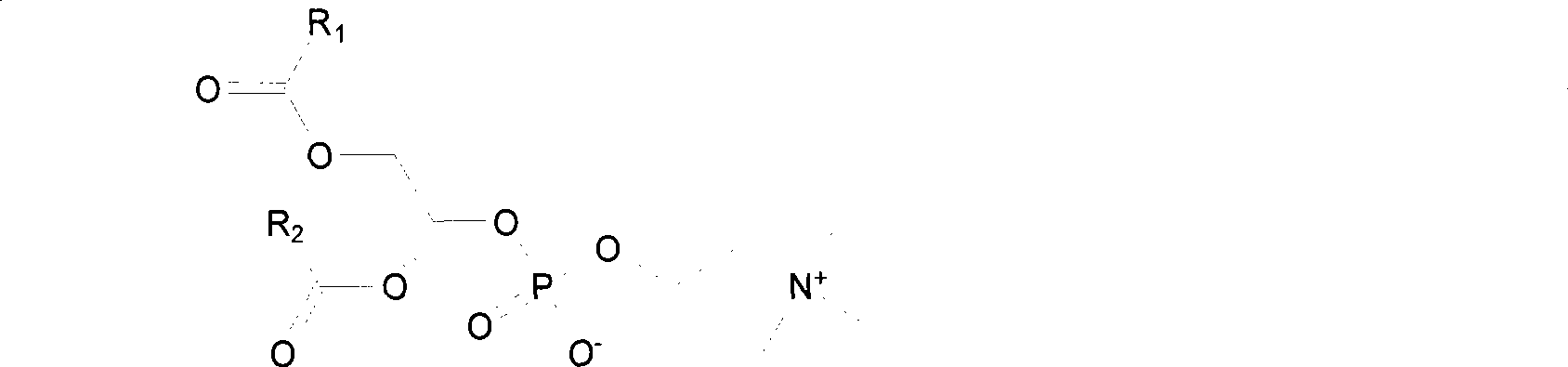
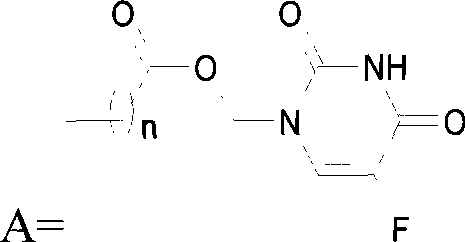
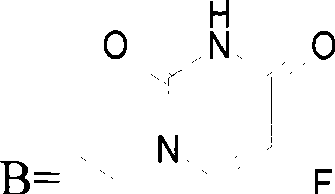
[0202] The carboxylic acid derivative of 5-fluorouracil - 1-acetic acid-5-fluorouracil, is condensed with 1-palmitoyloxy-sn-glycerol-phosphatidylcholine to obtain 5-fluorouracil-sn 2 - Phosphatidylcholine copolymer: 1-palmitoyloxy-2-(5-fluorouracil-1-acetoxy)-sn-glycerol-phosphatidylcholine.
[0203] Carboxylate derivatives of 5-fluorouracil——succinic acid mono[(5-fluoro-2,4-dioxo-3,-dihydro-2H-pyrimidin-1-yl)-methyl]ester, with 1 -palmitoyloxy-sn-glycerol-phosphatidylcholine condensation to give 5-fluorouracil-sn 2 - Phosphatidylcholine copolymer: 1-palmitoyloxy-2-{[(5-fluoro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-methoxycarbonyl ]-propionyloxy}-sn-glycerol-phosphatidylcholine.
[0204] Its chemical synthesis route is as follows:
[0205]
[0206] (2) 5-fluorouracil-sn 2 - Content control of phosphatidylcholine copolymer
[0207] The present invention adopts high perform...
Embodiment 1
[0245] The synthesis of embodiment 1,1-acetic acid-5-fluorouracil (1)
[0246] Weigh 0.03mol (3.9g) of 5-fluorouracil, 0.02mol potassium carbonate (1g), and 0.5g potassium iodide, put them into a 100ml reaction bottle, add 70ml dimethylsulfoxide (DMSO for short), and heat in an oil bath. Initially insoluble, it becomes cloudy after a period of heating. When the temperature rose to 70°C, 0.03 ethyl chloroacetate was added dropwise, and reacted overnight in an oil bath at 70°C. During the period, samples were taken at 10h, 12h, 14h, 16.5h, and 18.5h to spot the plate (TLC plate paved with GF254, with ethyl acetate:petroleum ether=3:1 as the developing solvent) to observe the 5- Formation of fluorouracil. When the reaction lasted for 18.5 hours, the reaction was terminated, and the reaction solution was evacuated at 90° C. with an oil pump, and DMSO was distilled off under reduced pressure. Dissolve the residue in 100ml of ethyl acetate (the residue is the dried product of a l...
Embodiment 2
[0248] Example 2, the synthesis of intermediate succinic acid mono[(5-fluoro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-methyl]ester (n=2)
[0249] Take 0.01mol 5-fluorouracil, 0.025mol 37% formaldehyde solution and stir at 60°C for reaction. After all the solids are dissolved, continue to stir for 1 hour. ), directly used in the next step of synthesis without purification.
[0250] Take 0.01 mol of intermediate (2), an appropriate amount of pyridine as a solvent, then add 0.01 mol of succinic anhydride, and stir at room temperature for reaction. After the reaction is completed, put the reaction mixture into an appropriate amount of water, extract with dichloromethane, wash with water, and dry over anhydrous sodium sulfate , filtered and concentrated to obtain a viscous liquid, purified by column chromatography to obtain intermediate succinic acid mono[(5-fluoro-2,-dioxo-3,4-dihydro-2H-pyrimidin-1-yl) with different side chain lengths )-methyl]ester (3), mp 153-156°C. Molecula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com