Quaternised chitosan-polycaprolactone grafting copolymer, preparation, reticulate membrann prepared by the copolymer and method for preparing the copolymer
A technology of quaternizing chitosan and polycaprolactone, which is applied in the direction of pharmaceutical formulations and medical preparations of non-active ingredients, etc., can solve the problems of difficult preparation of cross-linked fiber mesh membranes, etc., and achieve a controllable drug release rate , enhanced antibacterial and bactericidal properties, good breathability and moisture retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The molecular weight is 5.2×10 5 10g of chitosan powder with a degree of deacetylation of 96.4% was dispersed in 200ml of dimethylformamide, 27.6g of phthalic anhydride was added, and reacted at 120°C for 8h. After removing the phthalic anhydride, 500 ml of absolute ethanol was added, and the collected precipitate was washed and dried with water and ethanol to prepare 100% amino-protected phthaloyl chitosan.
[0025] Treat 2.0g of phthaloyl chitosan with high-purity nitrogen in the reactor for 30min, add 2.0g of caprolactone and 4ml of toluene, and add 2-ethylhexanoic acid equivalent to 1mol% of caprolactone monomer Stannous, reacted at 100°C for 24 hours under the protection of nitrogen. The reaction product was extracted with acetone for 24 hours in a Soxhlet extractor to obtain phthaloyl chitosan-polycaprolactone graft copolymer. Disperse 2.0g of phthaloyl chitosan-polycaprolactone graft copolymer in 20ml of dimethylformamide, add 4ml of hydrazine hydrate and react...
Embodiment 2
[0032] The molecular weight is 5.2×10 5 , the degree of deacetylation is 96.4% of 10g chitosan powder and 18.4g phthalic anhydride reacted in 200ml dimethylformamide, prepared into 100% amino-protected phthalic anhydride through the same method as in Example 1 acyl chitosan.
[0033] Treat 2.0g of phthaloyl chitosan with high-purity nitrogen in the reactor for 30min, add 4.0g of caprolactone and 6ml of toluene, and add 2-ethylhexanoic acid equivalent to 1mol% of caprolactone monomer Stannous, obtained chitosan-polycaprolactone graft copolymer 1.63g through the method identical with embodiment one preparation, polycaprolactone content 34.6wt%.
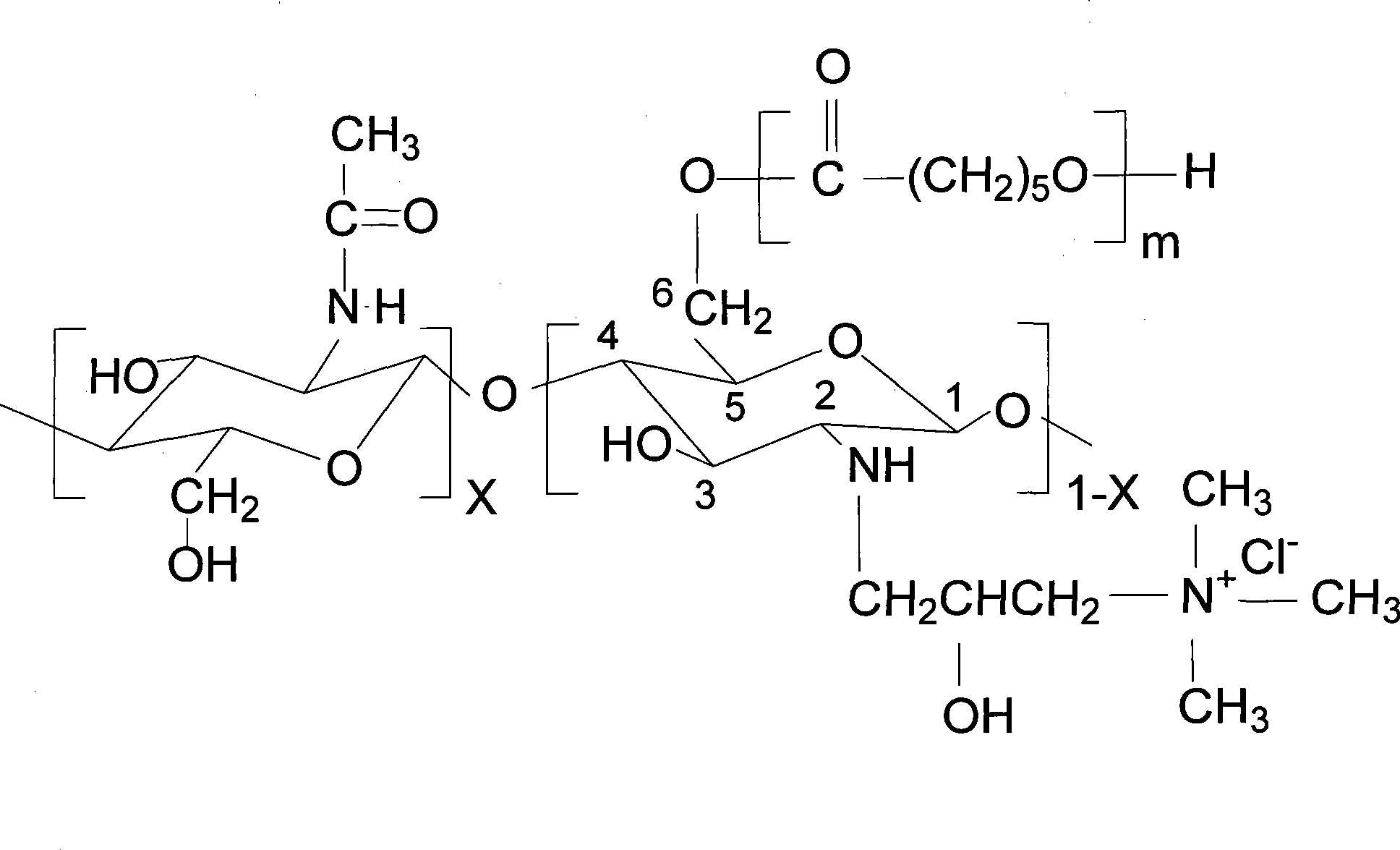
[0034] 3.0g chitosan-polycaprolactone graft copolymer fine powder and 8.5ml glycidyl trimethylamine chloride are added in the 35ml water phase and obtain quaternized chitosan-polycaprolactone through the method identical with embodiment one Graft copolymer 4.87g, quaternized side chain substitution degree 57.4%. The chemical structur...
Embodiment 3
[0040] The molecular weight is 5.2×10 5 , the degree of deacetylation is 96.4% of 10g chitosan powder and 23g phthalic anhydride react in 200ml dimethylformamide, prepare 100% amino-protected phthaloyl through the same method as Example 1 Chitosan.
[0041] Treat 2.0g of phthaloyl chitosan with high-purity nitrogen in the reactor for 30min, add 4.0g of caprolactone and 6ml of toluene, and add 2-ethylhexanoic acid equivalent to 1mol% of caprolactone monomer Stannous, prepared by the same method as in Example 1 to obtain 1.58 g of chitosan-polycaprolactone graft copolymer, and the content of polycaprolactone is 33.7wt%.
[0042] 3.0g chitosan-polycaprolactone graft copolymer fine powder and 10ml glycidyl trimethylamine chloride are added in 40ml water phase and obtain quaternization chitosan-polycaprolactone grafting through the method identical with embodiment one Copolymer 5.36g, quaternized side chain substitution degree 67.2%. The chemical structural formula of its quater...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average thickness | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com