Mannan-containing ligand and preparation method and application thereof
A mannan and ligand technology, applied in the field of chemistry, can solve the problems of limiting the application of nanocarriers, lack of specific recognition performance, toxic and side effects, etc., and achieve the effects of concentrated particle size distribution, round and smooth surface morphology, and extended shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1. Synthesis of the graft polymer (Mannan-PE) of mannan and phosphatidylethanolamine:
[0050] Mannan (100 mg) was dissolved in sodium hydroxide solution (3 mol / L, 1 mL), and stirred for 10 minutes to make it alkaline. Chloroacetic acid (16%, 1.2 mL) was then added and stirred in an oil bath at 55°C for 7 hours, then hydrochloric acid (1 mol / L) was added to adjust the pH to 2-3 to obtain carboxymethylated mannan. After carboxymethylated mannan was dissolved in dimethyl sulfoxide (DMSO), phosphatidylethanolamine (5 mg) which was also dissolved in dimethyl sulfoxide was added under stirring, and dimethyl sulfoxide was added dropwise under ice-bath conditions. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) (12 mg) dissolved in sulfone (adding an equimolar amount of triethylamine to it), stirred in an ice bath for one hours, then stirred at room temperature for 48 hours. Dimethyl sulfoxide was removed by rotary evaporation, the precipitate w...
Embodiment 2
[0053] Embodiment 2. Preparation of solid lipid nanoparticle suspension
[0054] Weigh 25 mg of stearic acid and 15 mg of lecithin, mix them and add 3 mL of acetone to ultrasonically dissolve them as the organic phase. Weigh 20 mg of cetyltrimethylammonium bromide (CTAB) and dissolve in 20 mL of double distilled water as the water phase. The organic phase was injected into the aqueous phase stirred at 600 r / min at a constant speed of 18 mL / h. Continue stirring at 400 r / min for 8 hours to completely volatilize the organic solvent, adjust the pH value to 7.2-7.4, and obtain a cationic solid lipid nanoparticle suspension.
Embodiment 3
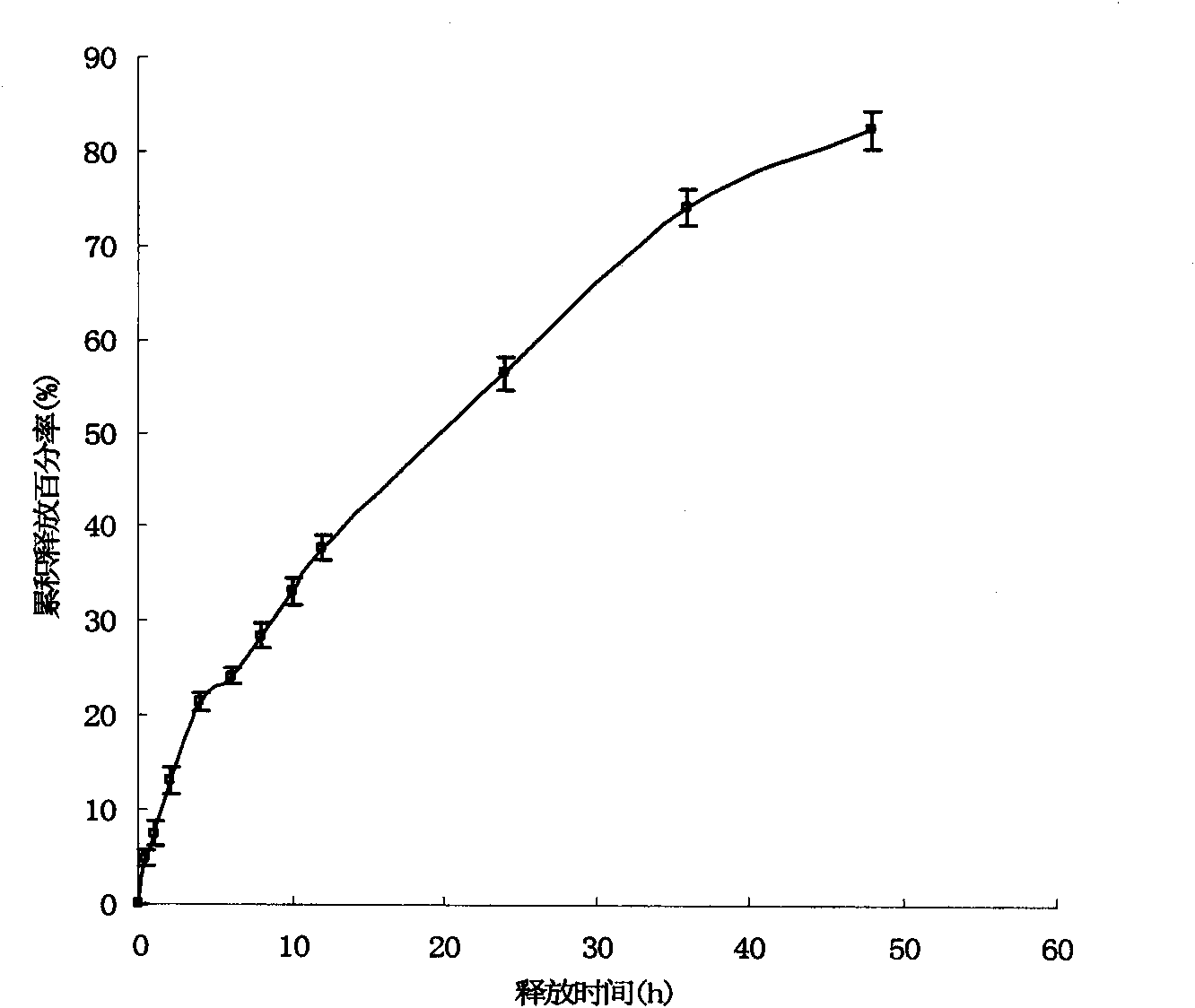
[0055] Embodiment 3: Research on the physical and chemical properties of solid lipid nanoparticle suspension
[0056] Get the solid lipid nanoparticle suspension in Example 2 and drop it onto a copper grid covered with a carbon film, let it stand for 2 minutes, blot the suspension with filter paper, then add dropwise a mass concentration of 2% phosphotungstic acid for negative staining for 2 minutes , observe the morphology of the nanoparticles under a transmission electron microscope and take pictures. Another appropriate amount of nanoparticle suspension was taken, and after appropriate dilution, the average particle size and polydispersity were measured with a Zetasizer 3000HS nanoparticle size analyzer. Then take an appropriate amount of nanoparticle suspension, and measure the zeta potential with a nanoparticle size analyzer after appropriate dilution.
[0057] The nanoparticles observed under the transmission electron microscope are spherical or quasi-spherical solid pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com