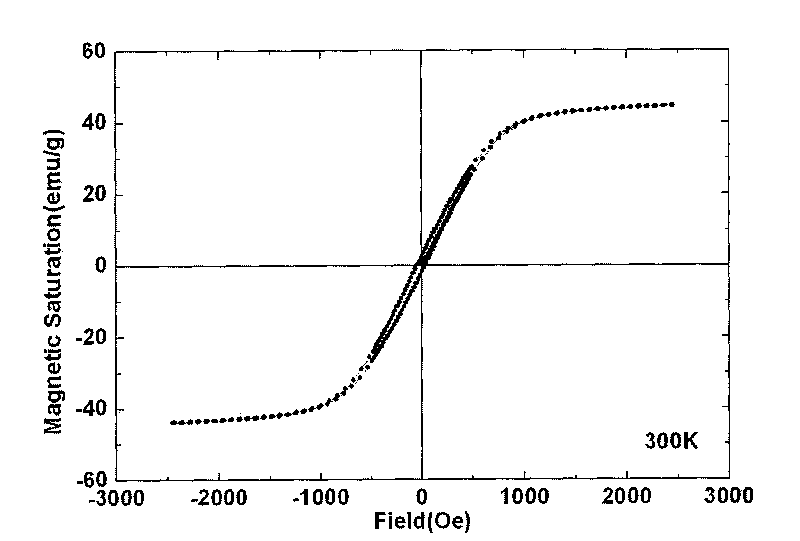
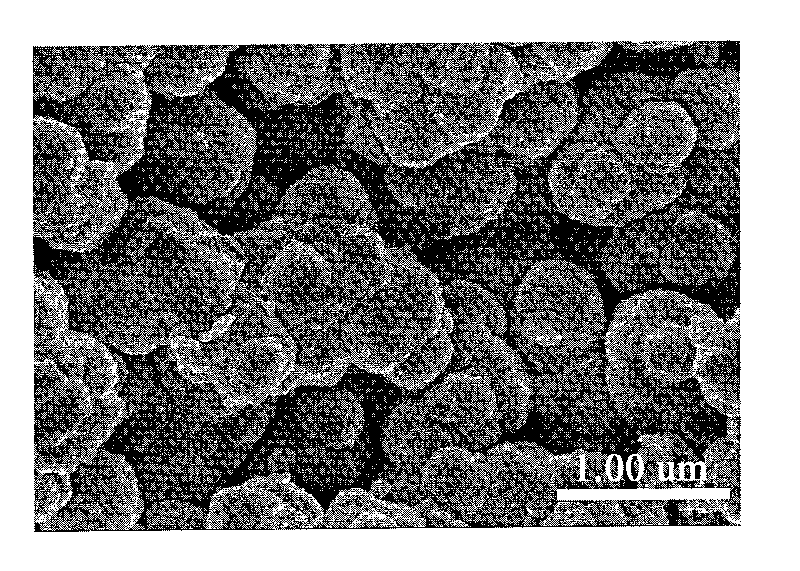
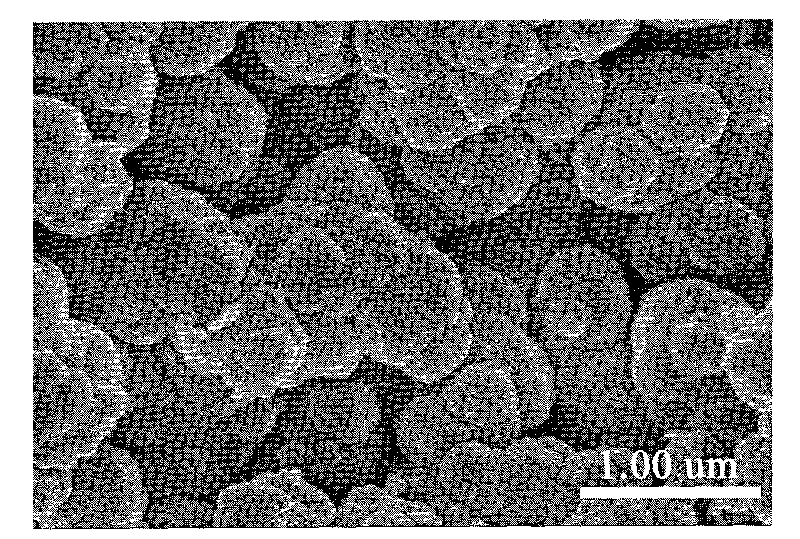High-magnetic heavy-metal ion adsorbent carrying conductive high molecules and preparation method thereof
A technology of conducting polymers and heavy metal ions, applied in non-metallic elements, chemical instruments and methods, adsorption water/sewage treatment, etc., can solve the problems of application limitation, inconvenient recycling, etc. Guaranteed effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh 1.5g of ferric chloride hexahydrate, 1g of polyethylene glycol 2000, and 3.6g of anhydrous sodium acetate and disperse them in 40ml of ethylene glycol, and stir vigorously for 60min. The precursor dispersion was transferred to a hydrothermal kettle, and the heating temperature was 200°C for 8 hours. ; Cool to room temperature, and through magnetic separation, the solvothermal product is washed with deionized water and absolute ethanol repeatedly 5 times. The solvothermal product was dried under vacuum at 80°C for 6h. Take 0.4 g of the above-mentioned dried terminal product and add it into 100 ml of isopropanol for dispersion, add 0.75 ml of ammonia water and 5.5 ml of deionized water at the same time, and stir vigorously for 30 min. 0.4 ml of tetrabutyl orthosilicate was added to the above dispersion, and then the entire hydrolysis reaction was carried out at 60° C. for 8 hours. The hydrolyzate was washed with water and alcohol for 5 times, and dried at 80° C. f...
Embodiment 2
[0036] Weigh 3g of ferric chloride hexahydrate, 5g of polyethylene glycol 2000, and 3.6g of anhydrous sodium acetate and disperse them in 40ml of ethylene glycol, and stir vigorously for 60min. The precursor dispersion was transferred to a hydrothermal kettle, and the heating temperature was 250° C. for 10 hours. The solvothermal product was repeatedly washed with deionized water and absolute ethanol 8 times. The solvothermal product was dried under vacuum at 80°C for 4 hours. Take 0.2 g of the above-mentioned dried terminal product and add it into 100 ml of isopropanol for dispersion, add 0.5 ml of ammonia water and 3 ml of deionized water at the same time, and stir vigorously for 30 min. 0.6 ml of tetrabutyl orthosilicate was added to the above dispersion, and then the entire hydrolysis reaction was carried out at 60° C. for 8 hours. The hydrolyzate was washed with water and alcohol for 8 times, and dried at 80° C. for 6 hours under vacuum condition. Add 2g of ferric chlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com