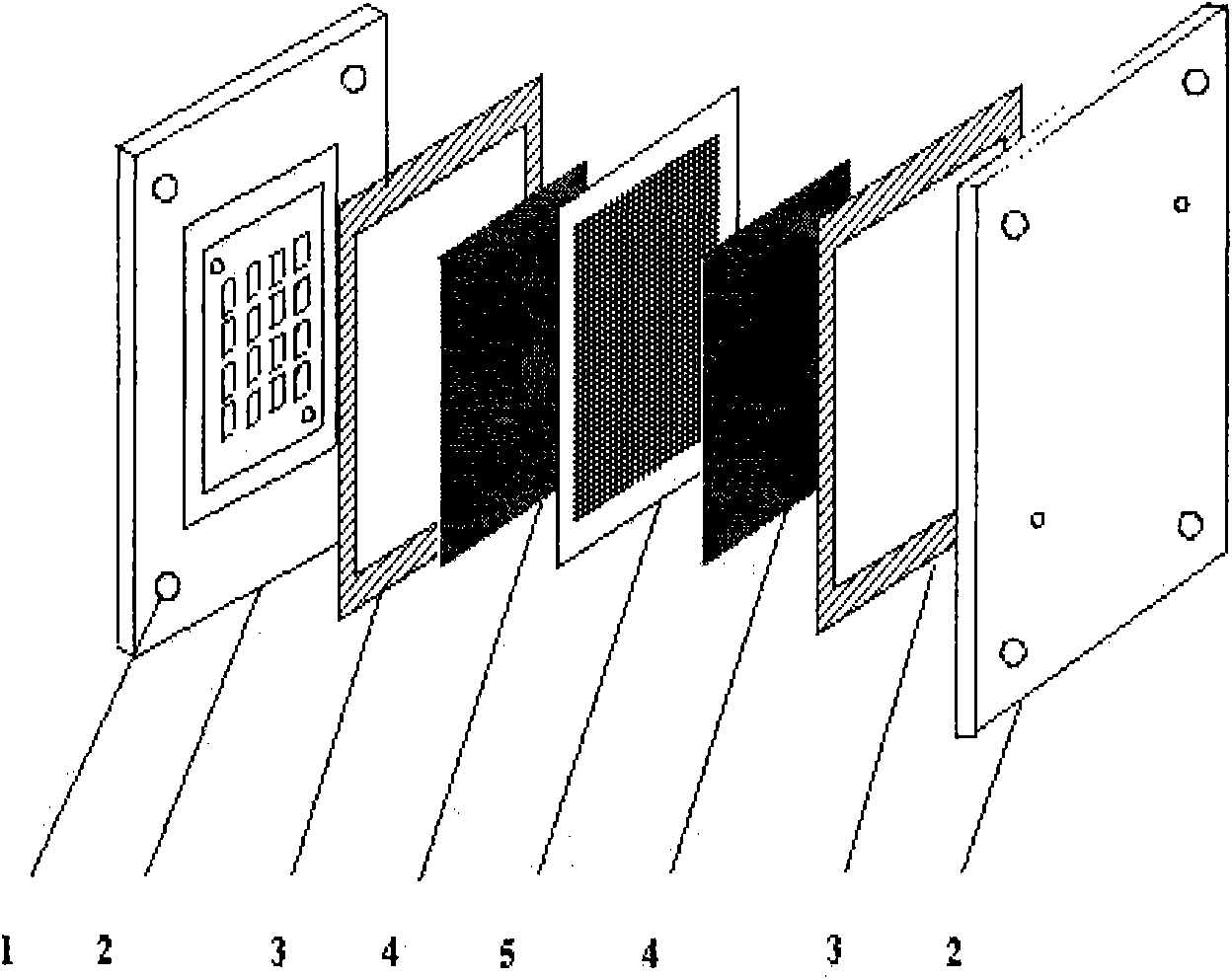
Catalyst using metal oxide as carrier for fuel cells and application thereof
A fuel cell and catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, battery electrodes, etc., can solve the problem of reducing the performance of URFC fuel cells and water electrolysis, and unable to fully occupy the catalytic layer. Space, catalyst increase the internal resistance of the catalytic layer, etc., to solve the effect of reducing the catalyst activity, improving the performance of the fuel cell, and being beneficial to the performance of the cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
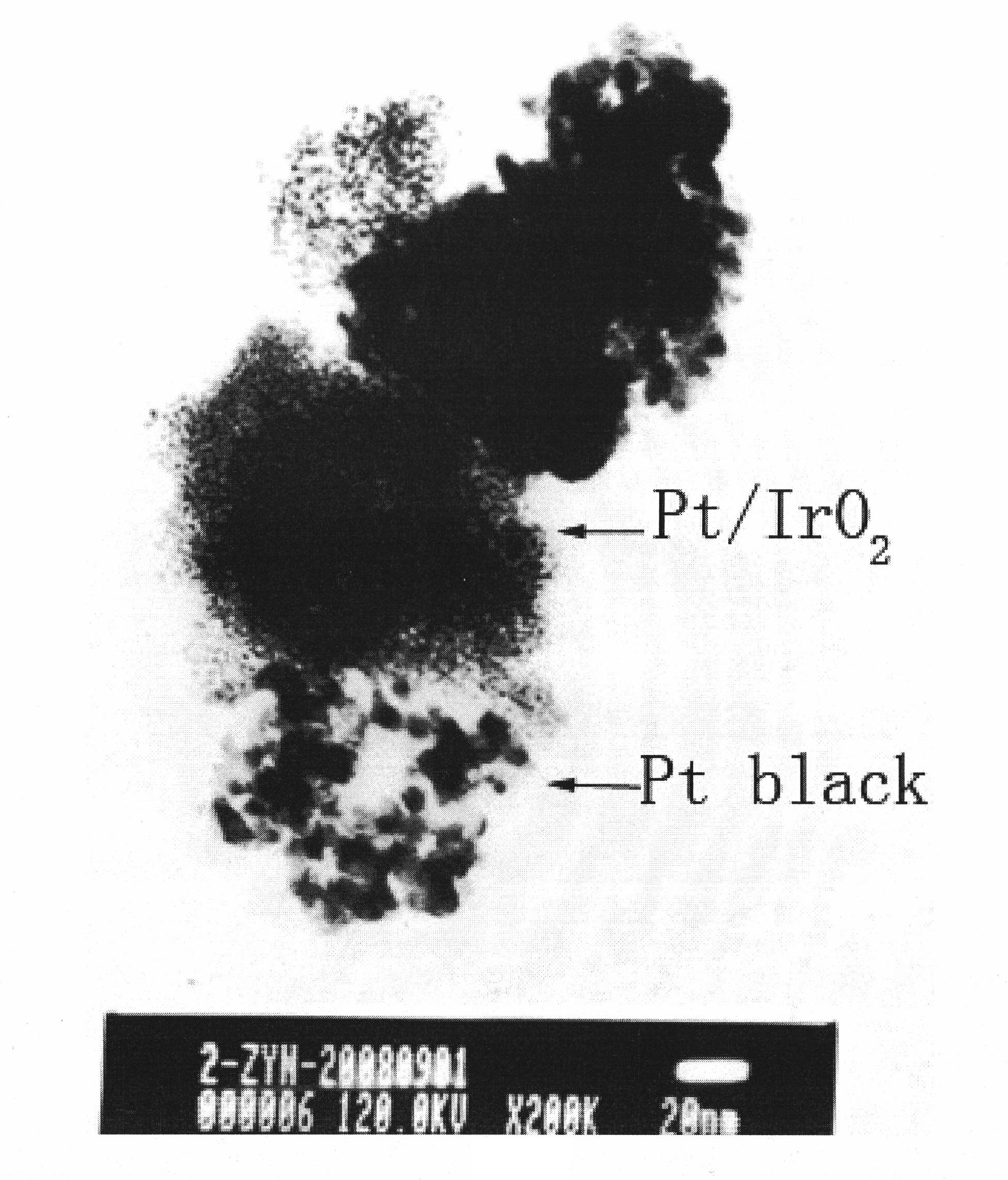
[0048] 20gNaNO 3 with 1gH 2 IrCl 6 Mixed, fully dissolved in water, fully stirred and mixed evenly, evaporated to dryness in a water bath at 80°C, and then transferred to an oven for drying. Grind the dried solid into a powder in a mortar. The obtained powdery solid is heat-treated at 450°C for 1 hour in an air atmosphere. After cooling down to room temperature naturally, the product is washed with deionized water and centrifuged repeatedly to remove impurities. ions, to obtain the oxide IrO with catalytic oxygen evolution reaction activity 2 .
[0049] 190mgIrO 2 Disperse in 200ml water, ultrasonic vibration to get IrO 2 Suspension, add chloroplatinic acid containing 10mg of platinum to it, after fully stirring and mixing, add Na 2 CO 3 Adjust the pH value of the solution to 8.5, add 2ml of formaldehyde, reflux for 1 hour in a heating environment at 80°C, cool to room temperature naturally, and repeatedly wash with deionized water to remove impurity ions by centrifugat...
Embodiment 2
[0062] 20gNaNO 3 with 1gH 2IrCl 6 Crystal hydrate (containing iridium 35.0%) and 0.645g SnCl 4 The crystalline hydrate (containing Sn33.5%) is mixed, fully dissolved in water, fully stirred and mixed evenly, evaporated to dryness in a water bath at 80°C, and then transferred to an oven for drying. Grind the dried solid into a powder in a mortar. The obtained powdery solid is heat-treated at 450°C for 1 hour in an air atmosphere. After cooling down to room temperature naturally, the product is washed with deionized water and centrifuged repeatedly to remove impurities. ions to obtain the oxide IrSnO with catalytic oxygen evolution function 4 .
[0063] 180mg IrSnO 4 Disperse in 200ml ethylene glycol, ultrasonic vibration to obtain IrSnO 4 To the suspension, add chloroplatinic acid containing 90 mg of platinum, stir and mix well, add NaOH to adjust the pH value of the solution to 13.5, reflux for 1 hour under a heating environment of 130 ° C, cool naturally to room tempera...
Embodiment 3
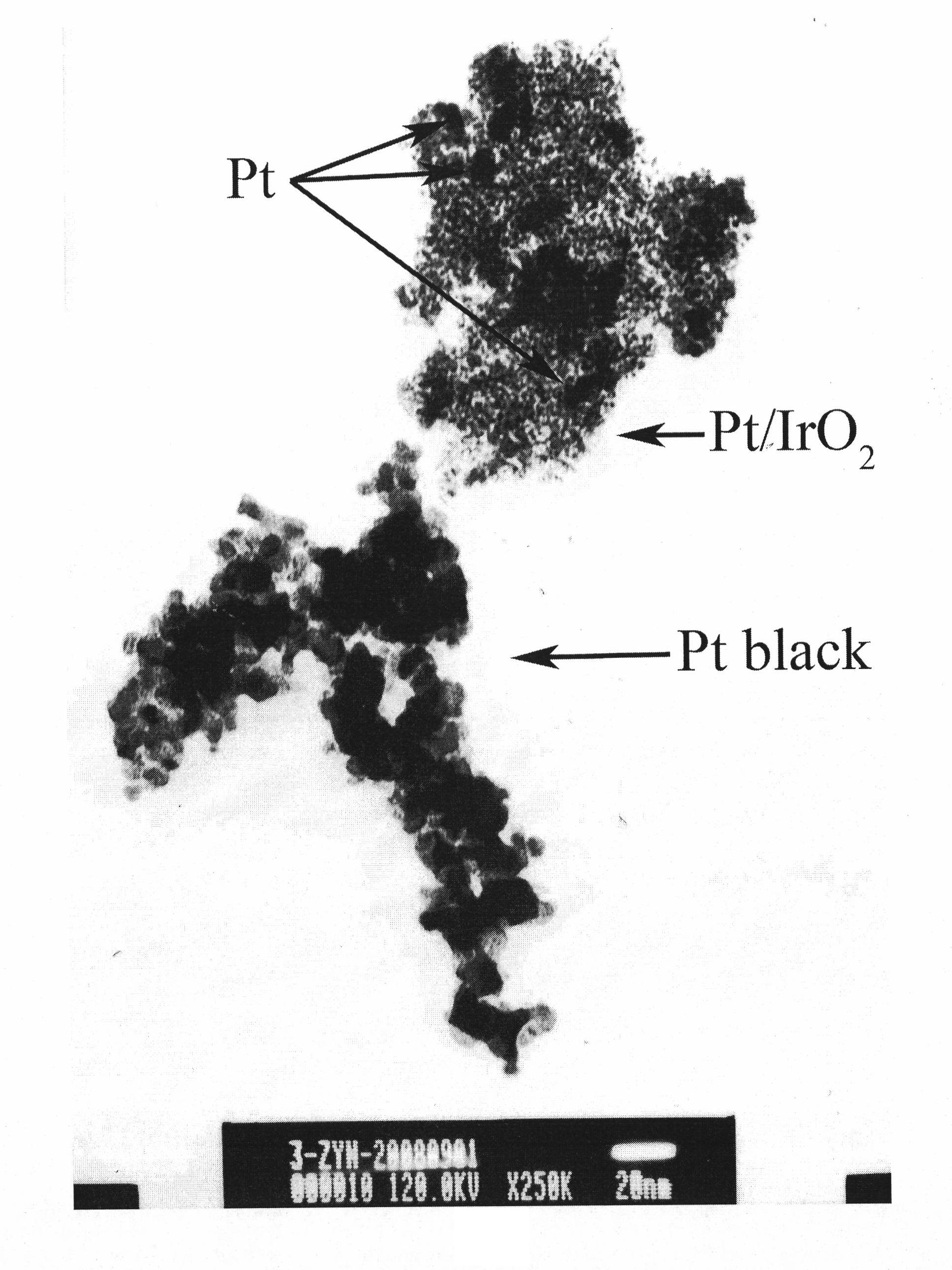
[0067] 1gH 2 IrCl 6 The crystalline hydrate is dissolved in 0.03mol / L NaOH solution, (Ir 4+ The molar ratio with NaOH is 1:9). At 40°C, pass through nitrogen protection, stir continuously for 4 hours, add 200 mg of ascorbic acid after cooling, cool to 5°C, keep the temperature for 30 minutes, use 0.1mol / L HClO 4 Adjust the pH value to 8 to obtain a light yellow precipitate. After settling for 30 hours, filter, wash, vacuum dry, and sinter in a tube furnace at 400°C for 1 hour to obtain IrO 2 .
[0068] 190mgIrO 2 Disperse in 200ml water, ultrasonic vibration to get IrO 2 Suspension, to which was added chloroplatinic acid containing 120mg of platinum, after fully stirring and mixing evenly, adding Na 2 CO 3 Adjust the pH value of the solution to 8.5, add 4ml of formic acid, reflux for 1 hour in a heating environment of 80°C, cool naturally to room temperature, use deionized water to wash repeatedly by centrifugal precipitation to remove impurity ions, and vacuum dry to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com