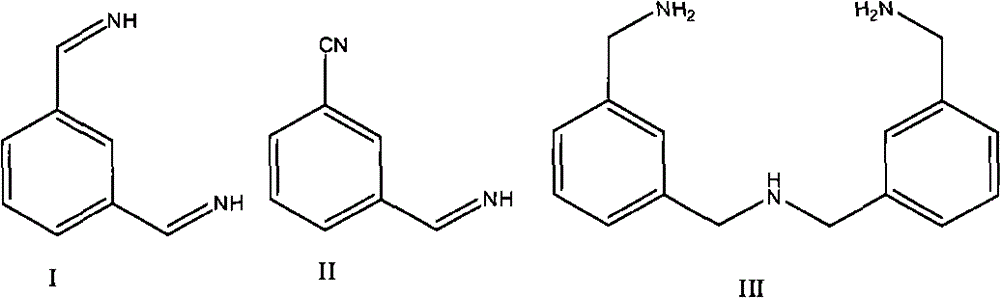
Method for preparing m-xylylenediamine
A m-xylylenediamine and isophthalonitrile technology, which is applied in the field of m-xylylenediamine preparation, can solve the problems of equipment manufacturing, high material requirements, no description of the main composition of impurities, and unsatisfactory XDA yield. , to achieve the effect of scientific process, compact equipment and mild operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] Catalyst preparation
[0032] catalyst one
[0033] Take by weighing 101g cobalt chloride hydrate, 28g nickel chloride hydrate, 11g titanium chloride hydrate, 20mg rhodium chloride hydrate, 3.6g manganese chloride hydrate, and 2.7g chromium chloride hydrate to form a solution. 100g of diatomaceous earth, after drying under an infrared lamp, be layered, and dried for later use.
[0034] catalyst two
[0035] Take by weighing 101g cobalt chloride hydrate, 28g nickel chloride hydrate, 11g titanium chloride hydrate, 20mg rhodium chloride hydrate, and 4.0g potassium molybdate to form a solution, and use the impregnation method to impregnate 100g of diatomaceous earth. After drying under the lamp, the bead is formed and dried for later use.
Embodiment 1
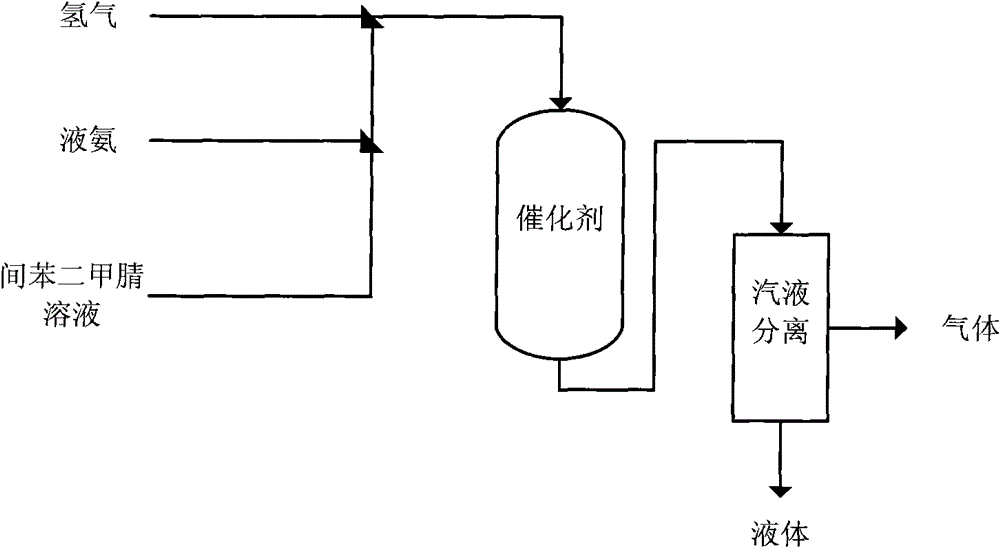
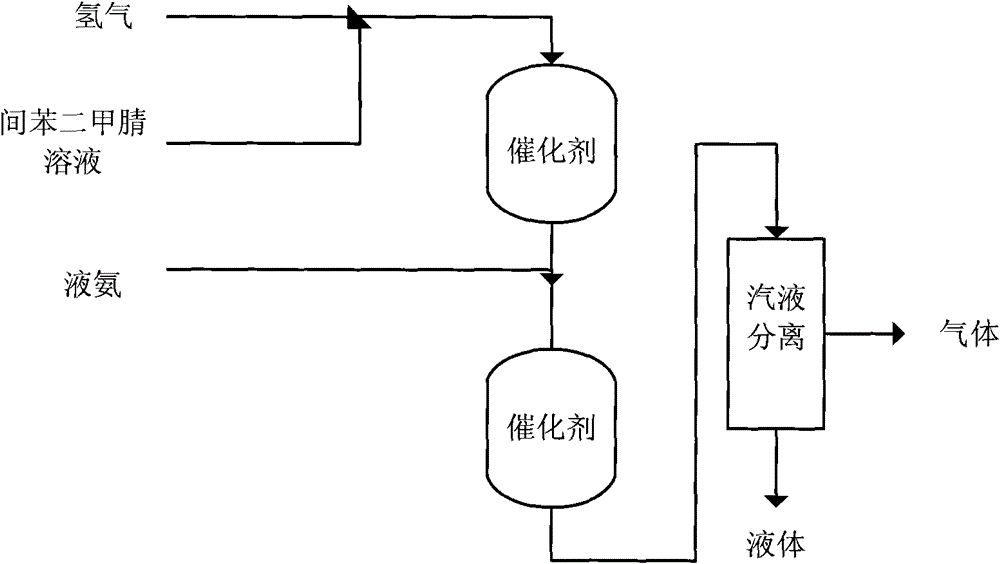
[0037]The hydrogenation reactor is a φ25mm×1500mm stainless steel tubular reactor, the reaction tube is filled with 80ml of catalyst 1 with a particle size of 0.6-1mm, and the bottom of the reaction tube is filled with inert quartz sand. The catalyst was reduced at 600° C. for 3 hours with a mixture of hydrogen and nitrogen (volume ratio 1:10) under normal pressure before use.
[0038] The mixed solution of isophthalonitrile (500g) and N-methylpyrrolidone (NMP) (2760ml) (the content of isophthalonitrile is 15%) is sent into the reactor from the top at a set speed, and the liquid ammonia (Flow rate is 47.5ml / min) and hydrogen (41.2 standard liters / H) are supplied from other two pipelines, and raw material enters the bed layer containing catalyst after mixing with hydrogen and liquid ammonia to carry out hydrogenation reaction. The reaction temperature is 75°C, the reaction pressure is 8Mpa, the addition amount of isophthalonitrile is kept at 9g / h, and the flow rate of liquid am...
Embodiment 2
[0040] According to the equipment and catalyst adopted in Example 1, isophthalonitrile (500 g) was dissolved in 2650 ml N-methylpyrrolidone (NMP), and hydrogenation reaction was carried out on a fixed bed, and hydrogenation reaction was carried out on a fixed bed. The reaction temperature was 85° C., the reaction pressure was 8 MPa, the addition amount of isophthalonitrile was kept at 13 g / h, and the hydrogen flow rate was controlled at 41.2 standard liters / H under the condition of no ammonia. The reaction solution was collected and analyzed, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com