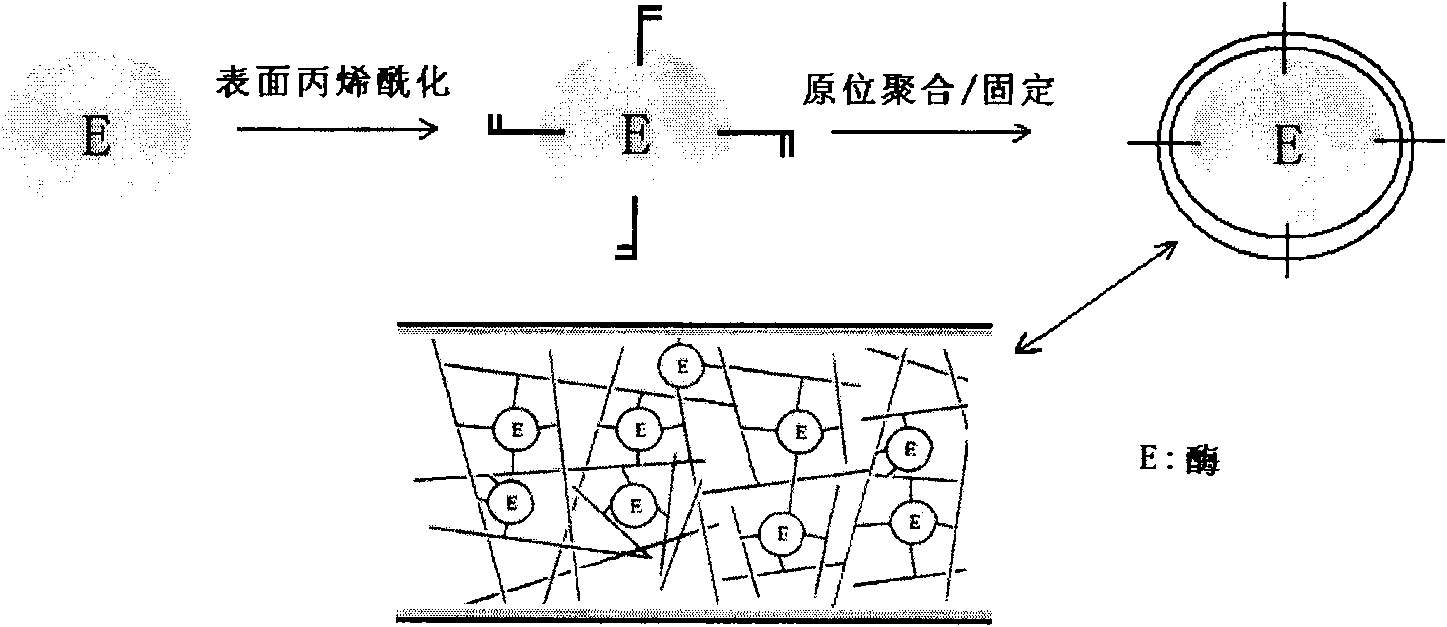
Preparation method of protein quick enzymolysis monolithic column through in situ polymerization and application thereof
An in-situ polymerization and protein technology, applied in the fields of analytical chemistry and proteomics, to achieve the effect of simple operation, simple and effective preparation method and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of capillary monolithic columns for rapid enzymatic digestion of proteins:
[0026] Capillary cleaning and activation: Quartz capillary (inner diameter: 250μm) is cut into 6cm sections, washed with methanol for 5 minutes, pure water for 5 minutes, 0.1mol / L sodium hydroxide for 30 minutes, pure water for 5 minutes, and 0.1mol / L hydrochloric acid 30 minutes, wash with pure water for 5 minutes, and methanol for 5 minutes to wash away impurities in the tube and expose the silanol on the inner wall. Use a peristaltic pump to fill the capillary with 50% γ-MAPS methanol solution, and react in the dark for 24 hours at room temperature, so that the inner wall of the capillary is connected with carbon-carbon double bonds.
[0027] Chemical modification on the surface of enzyme molecules: 10 mg of Trypsin solution is freshly dissolved in 5 mL of 100 mmol / L boric acid buffer solution with pH=9.3, and a certain mass of benzamide is added to make the final concentration 0...
Embodiment 2
[0030]Enzymolysis and mass spectrometric determination of the standard protein horse myoglobin (Myo) by the monolithic column for rapid enzymatic digestion of proteins:
[0031] 50 μg of MYO was dissolved in 50 μL of 25 mM ammonium bicarbonate solution, and then passed through the enzymatic monolithic column at a flow rate of 3 μL / min driven by a peristaltic pump. The volume of each spot is about 1 μL, and when it shrinks to about 0.5 μL by rapid air-drying at room temperature, add 0.4 μL of matrix solution (α-cyano-4-hydroxycinnamic acid CHCA) to each spot, and mix and air-dry used for identification by mass spectrometry. In MALDI-TOF / TOF (4700 Proteomics Analyzer, Applied Biosystems); laser is Nd-YAG laser, wavelength 355nm, laser pulse frequency 200Hz; acceleration voltage 20KV; positive ion mode, reflective TOF detection. Depend on Figure 4A As shown, the main characteristic peptide peaks were all detected, and the intensity was strong, indicating that the protein was e...
Embodiment 3
[0033] The concentration of preparing bovine serum albumin (BSA) is 1 μ g / μ L, preparation method and other conditions are the same as example 2, repeat above-mentioned enzymatic hydrolysis and mass spectrometry experiment, the result is as follows Figure 4B . Similarly, the main characteristic peptide peaks of BSA were detected with strong intensity, indicating that the protein was effectively hydrolyzed after the capillary enzymolysis monolithic column.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com