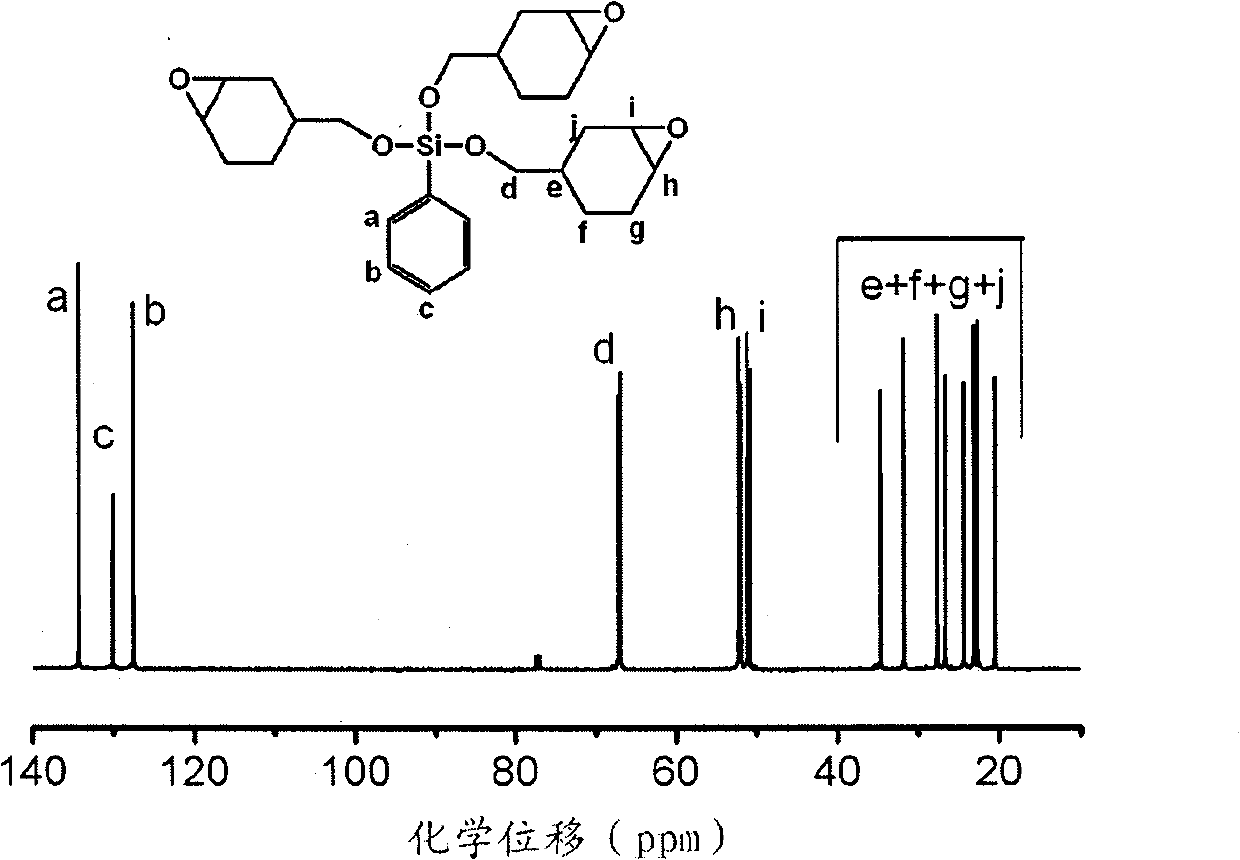
Cation ultraviolet curing group-containing silicon oxide compound and preparation method thereof
A silicon-oxygen compound and light-curing technology, which is applied in the fields of compounds of group 4/14 elements of the periodic table, chemical instruments and methods, organic chemistry, etc., can solve difficult control of process conditions, unstable product quality, intense condensation reaction, etc. problem, to achieve the effect of easy control of process conditions, convenient industrial production, and low content of organic volatiles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 19.8g (0.1mol) phenyltrimethoxysilane, 100.8g (0.9mol) 3-cyclohexene-1-methanol and 0.28g (0.001mol) tetraisopropyl titanate were placed in a round bottom flask, React at 100°C under stirring until the methoxy peak in the H NMR spectrum disappears; after removing excess 3-cyclohexene-1-methanol under reduced pressure, add 80 mL of toluene to dilute the reaction solution, and then use 5% tartaric acid aqueous solution, Washing with 5% sodium bicarbonate aqueous solution and distilled water, and finally drying the organic phase with anhydrous sodium sulfate, and the transparent liquid obtained after vacuum distillation is silicon-containing olefin.
[0045] Add 62.1g (0.36mol) m-chloroperoxybenzoic acid and 200mL methylene dichloride in the flask equipped with mechanical stirring, thermometer and constant pressure dropping funnel, after mixing uniformly, add dropwise the silicon-containing olefin that above-mentioned reaction makes, in After reacting at 25°C for 24 hours,...
Embodiment 2
[0052] 13.6g (0.1mol) methyltrimethoxysilane, 37.3g (0.3mol) 5-norbornene-2-methanol and 0.28g (0.001mol) tetraisopropyl titanate were placed in a round bottom flask, React under stirring at 100°C until the methoxy peak in the H NMR spectrum disappears; then add 80 mL of toluene to dilute the reaction solution, then wash with 5% tartaric acid aqueous solution, 5% sodium bicarbonate aqueous solution and distilled water, and finally the organic phase Dry with anhydrous sodium sulfate and distill under reduced pressure to obtain a transparent liquid that is silicon-containing olefin.
[0053] In the flask equipped with mechanical stirring, thermometer and constant pressure dropping funnel, add 62.1g (0.36mol) m-chloroperoxybenzoic acid and 160mL dichloromethane and 40mLN, the mixed solvent of N-dimethylformamide composition, mix After uniformity, add the silicon-containing olefin prepared by the above reaction dropwise, and after reacting at 25°C for 24 hours, filter to remove th...
Embodiment 3
[0057] Put 15.2g (0.1mol) of methyl orthosilicate, 245g (2.4mol) of ethylene glycol monoallyl ether and 0.28g (0.001mol) of tetraisopropyl titanate in a round-bottomed flask, under stirring Vacuumize and react at 50°C until the methoxy peak in the H NMR spectrum disappears; then first add 80mL of toluene to dilute the reaction solution, then wash with 5% tartaric acid aqueous solution, 5% sodium bicarbonate aqueous solution and distilled water, and finally wash the organic phase with Dry over anhydrous sodium sulfate and distill under reduced pressure to obtain a transparent liquid that is silicon-containing olefin.
[0058] Add 82.8g (0.48mol) m-chloroperoxybenzoic acid and 250mL methylene dichloride in the flask that mechanical stirring, thermometer and constant pressure dropping funnel are housed, after mixing uniformly, add dropwise the silicon-containing olefin that above-mentioned reaction makes, in After reacting at 25°C for 24 hours, the white solid was removed by filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com