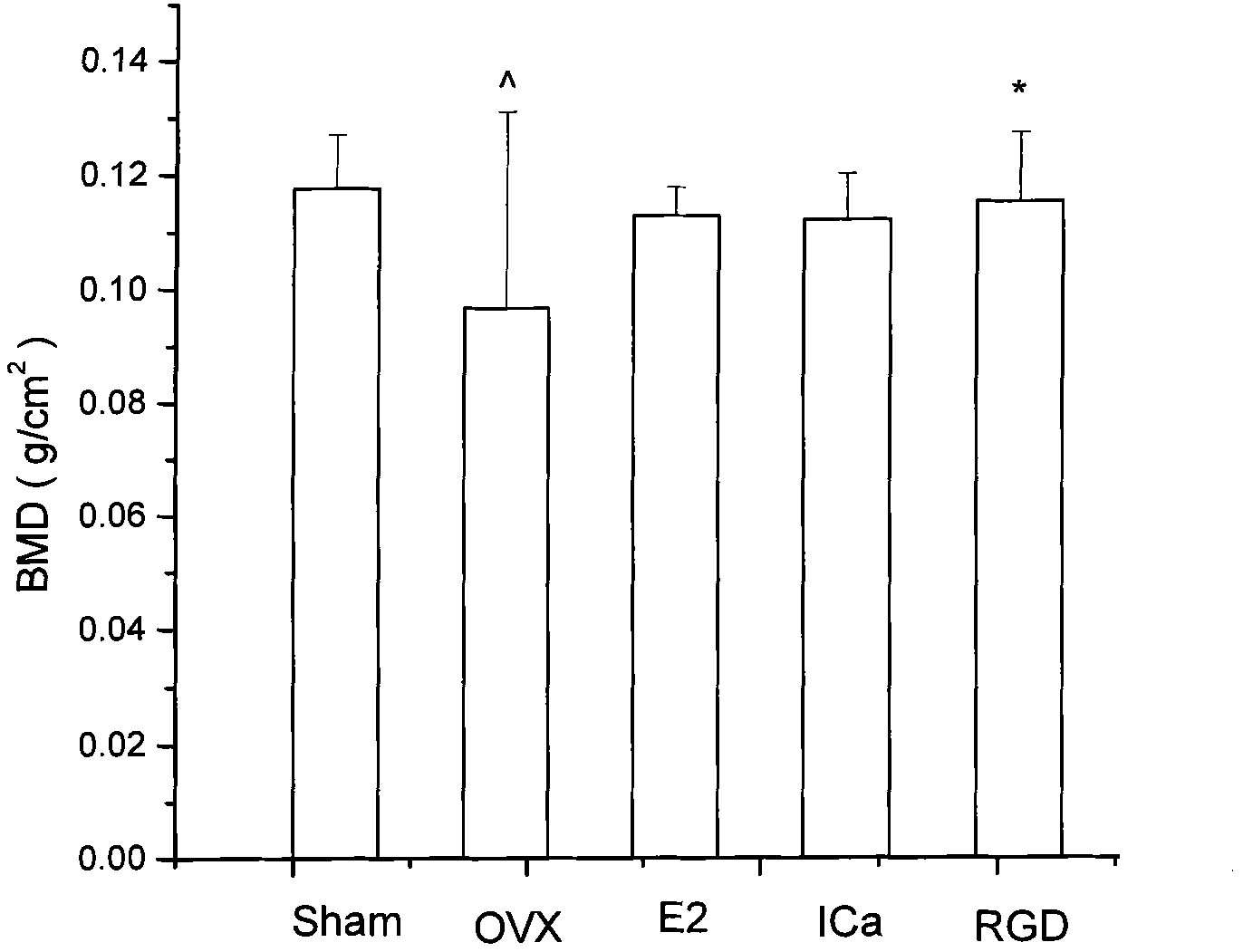
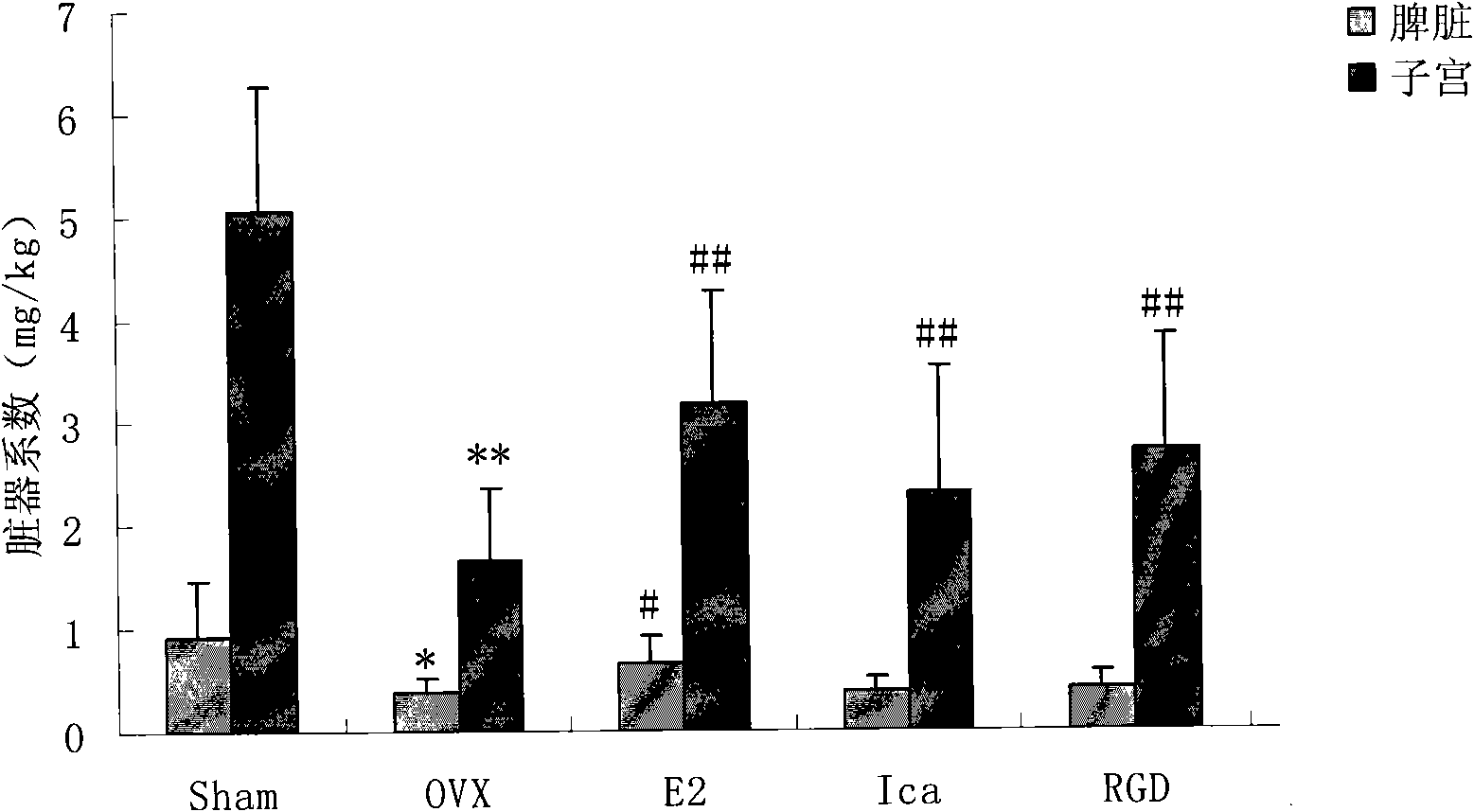
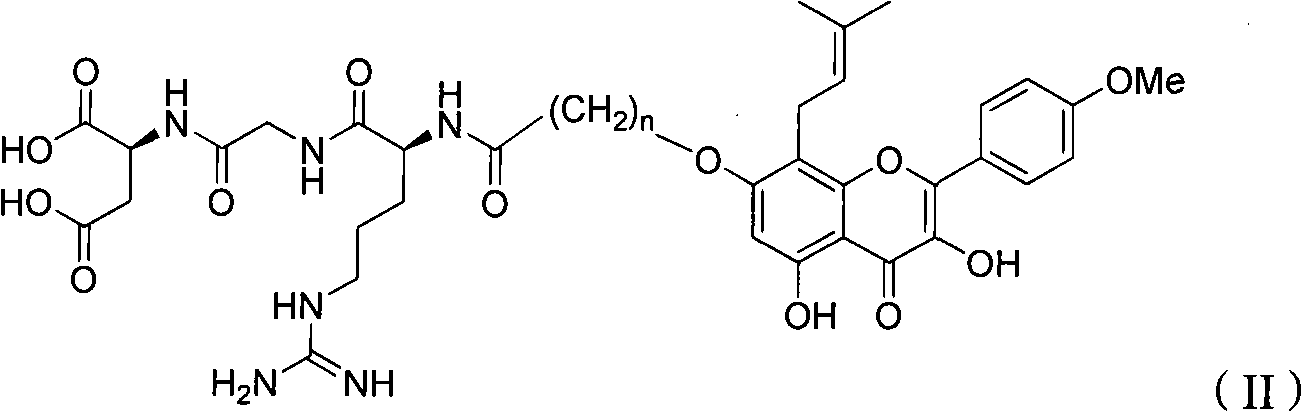
RGD peptide-epimedium flavone combination, preparation method and application thereof
A technology of Huo flavonoid conjugates and epimedium, which is applied in the field of pharmaceutical compounds, can solve the problems of lack of theoretical guidance and unified standards, shortage, confusion of traditional Chinese medicine prescriptions, etc., and achieve the effects of high yield, easy product and fast synthesis speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of N-4-(7-O-Epimedium flavonoids) butyryl-RGD (n=3 in general formula II):
[0054] synthetic N G -Mesitylenesulfonyl-RGD dimethyl ester (Arg(Mts)-Gly-Asp(OMe) 2 ):
[0055] N G -Mesitylenesulfonyl-N-benzyloxycarbonyl-RGD dimethyl ester (Cbz-Arg(Mts)-Gly-Asp(OMe) 2 , Compound III) (5.0g, 7.2mmol) was dissolved in 400ml of methanol, 10% carbon-supported palladium catalyst (0.5g) was added, hydrogen was introduced and stirred at room temperature for 24h, the catalyst was removed by diatomaceous earth filtration, and the filtrate was evaporated under reduced pressure Solvent yielded 3.6 g of white solid N G -Mesitylenesulfonyl-RGD dimethyl ester (Arg(Mts)-Gly-Asp(OMe) 2 ), yield 89%;
[0056] Synthesis of N-(4-bromobutyryl)-N G -Mesitylenesulfonyl-RGD dimethyl ester (Br(Br(CH 2 ) 3 CO-Arg(Mts)-Gly-Asp(OMe) 2 ):
[0057] 4-Bromobutyric acid (1.1g, 6.5mmol) was dissolved in 200ml of dry dichloromethane, and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide s...
Embodiment 2
[0063] Example 2 Synthesis of N-11-(7-O-Epimedium flavonoids)undecanoyl-RGD (n=10 in general formula II):
[0064] synthetic N G -Mesitylenesulfonyl-RGD dimethyl ester (Arg(Mts)-Gly-Asp(OMe) 2 ): with embodiment 1 step 1).
[0065] Synthesis of N-(11-bromoundecanoyl)-N G -Mesitylenesulfonyl-RGD dimethyl ester (Br(Br(CH 2 ) 10 CO-Arg(Mts)-Gly-Asp(OMe) 2 ):
[0066] 11-Bromoundecanoic acid (530 mg, 2.0 mmol) was dissolved in 100 ml of dry dichloromethane, and benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate (PyBOP ) (1.0g, 2.0mmol), 1-hydroxy-7-azobenzotriazole (HOAt) (50mg, 0.4mmol), N G -Mesitylenesulfonyl-RGD dimethyl ester (Arg(Mts)-Gly-Asp(OMe) 2 ) (1.1g, 2.0mmol), N, N-diisopropylethylamine (0.7ml, 4mmol), stirred at room temperature for 48 hours after adding, then added 100ml dichloromethane, and used 200ml 1mol / liter Wash twice with hydrochloric acid, then wash with saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride, dr...
Embodiment 3
[0073] Example 3 Synthesis of N-21-(7-O-Epimedium flavonoids) eicosanoyl-RGD (n=20 in general formula II):
[0074] synthetic N G -Mesitylenesulfonyl-RGD dimethyl ester (Arg(Mts)-Gly-Asp(OMe) 2 ): with embodiment 1 step 1).
[0075] Synthesis of N-(21-bromoicocoyl)-N G -Mesitylenesulfonyl-RGD dimethyl ester (Br(Br(CH 2 ) 20 CO-Arg(Mts)-Gly-Asp(OMe) 2 ):
[0076] 21-Bromoeicosanoic acid (810mg, 2.0mmol) was dissolved in 100ml of dry dichloromethane, and benzotriazole-N,N,N',N'-tetramethylurea was added to the solution in sequence Hexafluorophosphate (HBTU) (1.1g, 3.0mmol), 6-chloro-1-hydroxybenzotriazole (Cl-HOBt) (140mg, 0.8mmol), N G -Mesitylenesulfonyl-RGD dimethyl ester (Arg(Mts)-Gly-Asp(OMe) 2 ) (1.1g, 2.0mmol), N, N-dimethylaminopyridine (DMAP) (600mg, 10mmol), stirred at room temperature for 24 hours after adding, then added 100ml dichloromethane, and used 200ml 1mol / liter Wash twice with hydrochloric acid, then wash with saturated aqueous sodium bicarbonate and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com