Method for synthesizing flame retardant hexaphenoxy cyclotriphosphazene
A technology of hexaphenoxycyclotriphosphazene and synthesis method, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., and can solve separation and purification troubles, quaternary ammonium salts or quaternary A large amount of phosphonium salt catalyst is used, the operation process is complicated, etc., and the separation and purification methods and steps are simplified, the catalytic reaction effect is good, and the preparation process is simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
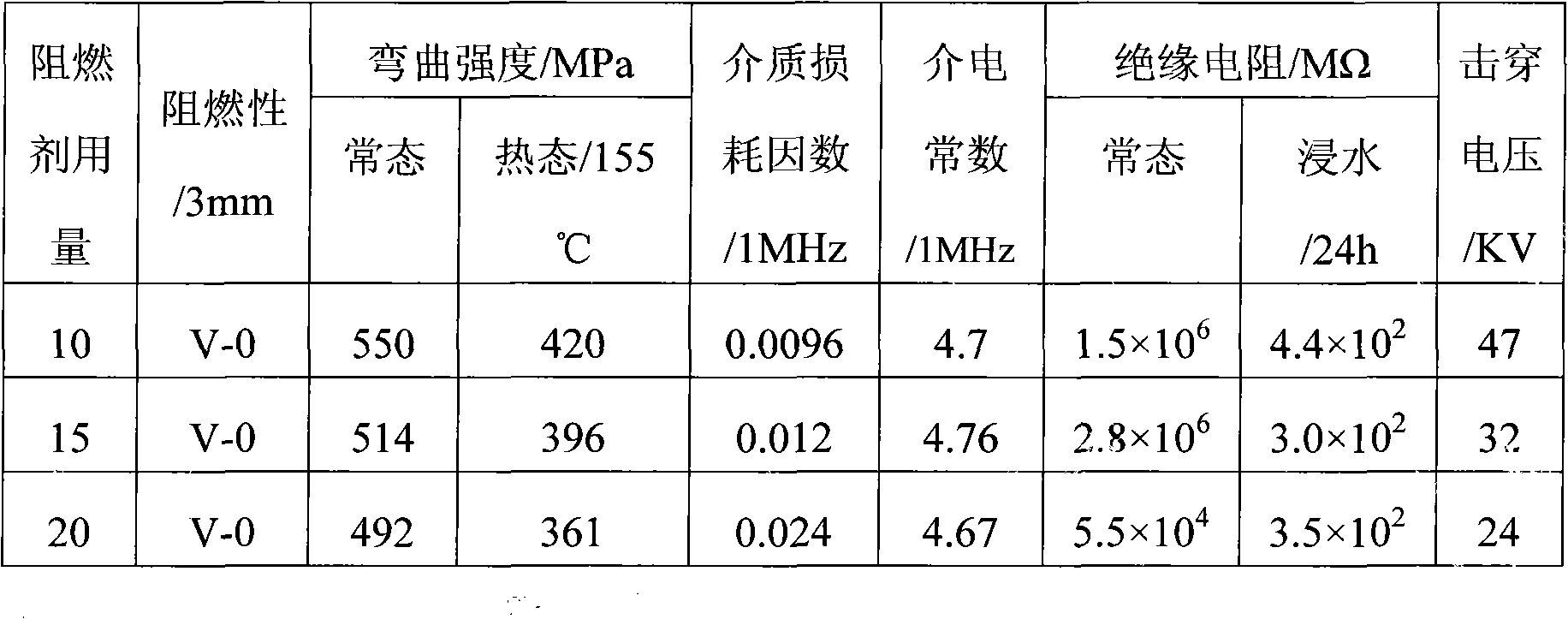
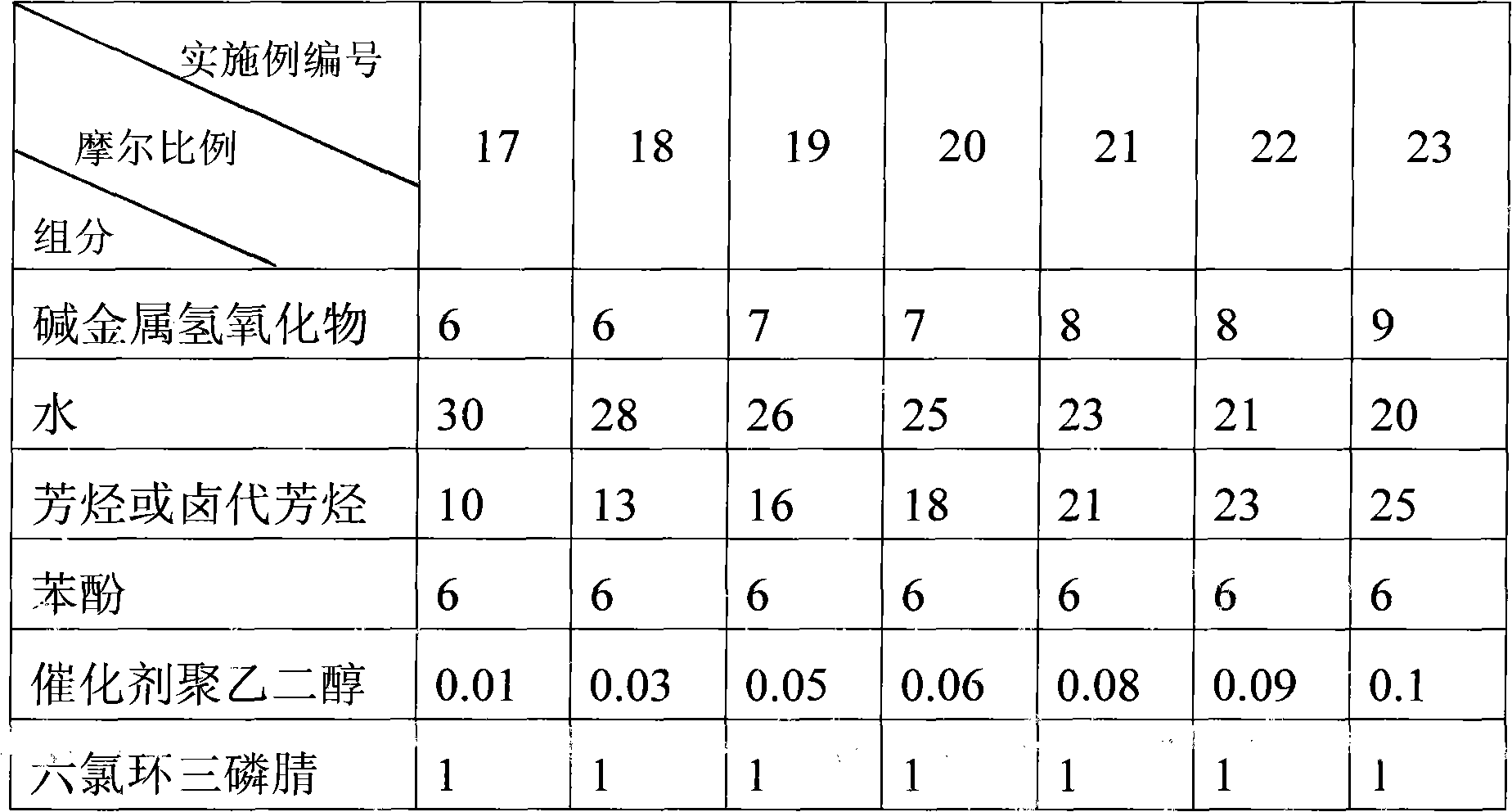
[0028] In a 500mL three-neck flask equipped with a stirrer, a thermometer, and a reflux condenser, add 33.6g (0.6mol) of potassium hydroxide, 36g of water, 230g of toluene, 56.4g (0.6mol) of phenol, 4g (0.01mol) of polyethylene Diol 400, 34.8g (0.1mol) hexachlorocyclotriphosphazene were added to the reactor in sequence, first maintained at 20°C to 30°C for 5 hours, then raised to 80°C to 90°C for 5 hours, and the system cooled down to Below 30°C, stand and separate layers. The brownish-yellow organic phase in the upper layer is washed with 300g of water for 3 times until neutral, and about 220g of toluene is recovered by distillation. The residue is poured out while it is hot, and 66.4g of white crystalline solid powder is obtained after cooling. That is the flame retardant hexaphenoxycyclotriphosphazene product with a yield of 95.8%.
Embodiment 2
[0030] Potassium hydroxide addition becomes 50.4g (0.9mol), and water yield becomes 54g, and other is with embodiment 1, omission, obtains product 66.6g, and yield is 96.1%.
Embodiment 3
[0032] Potassium hydroxide is replaced with sodium hydroxide, and addition becomes 24g (0.6mol), and other is with embodiment 1, omission, obtains product 65.9g, and yield is 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com