Clostridium welchii disease resistant transgenic plant vaccine and preparation method thereof
A technology for transgenic plants, Clostridium wilfordii disease, applied in the fields of botanical equipment and methods, biochemical equipment and methods, antibacterial drugs, etc. Bacteria environmental pollution and other problems, to achieve the effect of improving effectiveness, improving immunity, and enriching nutrients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
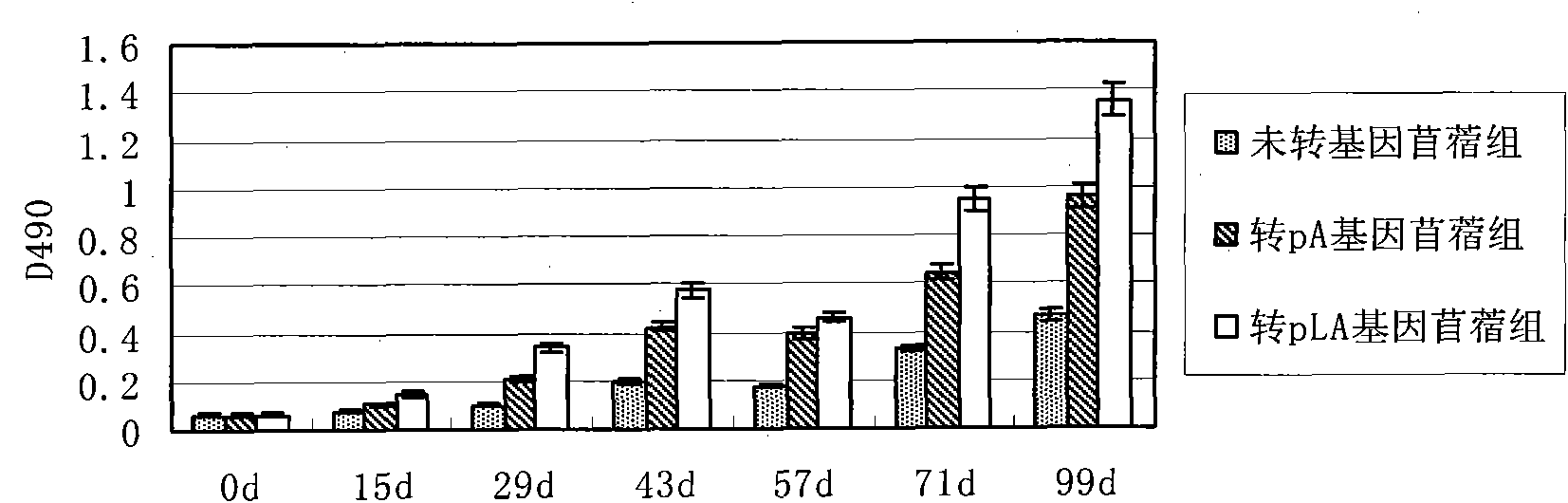
Image
Examples
Embodiment 1
[0047] A method for making a transgenic plant vaccine against Clostridium welchiiosis, the method comprising:
[0048] (1) Using a plasmid or disease material encoding Clostridium welchii α toxin as a template, PCR or RT-PCR is used to amplify the Clostridium welchii α toxin gene, and the obtained Clostridium welchii α toxin gene and Ti plasmid Carry out enzyme digestion respectively, recover the target gene and plant expression vector fragments, connect and transform Escherichia coli, carry out enzyme digestion, PCR and sequencing identification on the recombinant Ti, transform Agrobacterium tumefaciens with the recombinant plasmid, and obtain positive recombinant Agrobacterium tumefaciens through screening and PCR identification pA: It was deposited in the China Center for Type Culture Collection on June 2, 2010, and the preservation number is: CCTCC NO: M 2010130.
[0049] (2) Plant seeds undergo vernalization, germination, and splicing to obtain explants, respectively infe...
Embodiment 2
[0054] A preparation method of an anti-clostridium welchiiosis transgenic plant vaccine, the method comprising:
[0055] (1) Using the plasmid or disease material containing the mucosal adjuvant Escherichia coli heat labile enterotoxin B subunit (LT-B) as a template, use PCR or RT-PCR to amplify the mucosal adjuvant LT-B gene to contain The plasmid or disease material encoding Clostridium welchii α-toxin is used as a template, and PCR or RT-PCR is used to amplify the α-toxin gene of Clostridium welchii, and the mucosal adjuvant LT-B gene and the Clostridium welchii Bacterial α-toxin gene fusion to obtain the fusion gene, the obtained fusion gene and Ti plasmid were digested separately, the target gene and plant expression vector fragments were recovered, ligated and transformed into Escherichia coli, and the recombinant Ti plasmid was identified by enzyme digestion, PCR and sequencing. The recombinant plasmid was transformed into Agrobacterium tumefaciens, and positive recombi...
Embodiment 3
[0062] 1 test material
[0063] 1.1 Plasmids and strains
[0064] Plasmid pET-30b-a contains the α-toxin gene sequence of Clostridium welchii and was constructed by our laboratory; plasmid pLT-b contains the gene sequence of Escherichia coli heat-labile enterotoxin B subunit (LT-B) and was constructed by Mr. Ge Junwei of our laboratory ; The plant expression vector p33EG contains the E12 promoter sequence, the Ω sequence that enhances translation, the Bar gene of resistance to PPT (Phosphinothricin) herbicide, the prokaryotic expression of NptⅡ (Km resistance) gene and Agrobacterium tumefaciens LBA 4404. Gifted by Professor Zhu Yanming of the college; Clostridium welchii type A (C57-1) was purchased from China Veterinary Drug Control Institute.
[0065] 1.2 Antibody preparations
[0066] Anti-clostridium welchii alpha toxin rabbit serum, mouse serum and anti-Escherichia coli heat-labile enterotoxin B subunit (LT-B) mouse serum were prepared by our laboratory; Anti-mouse IgA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com