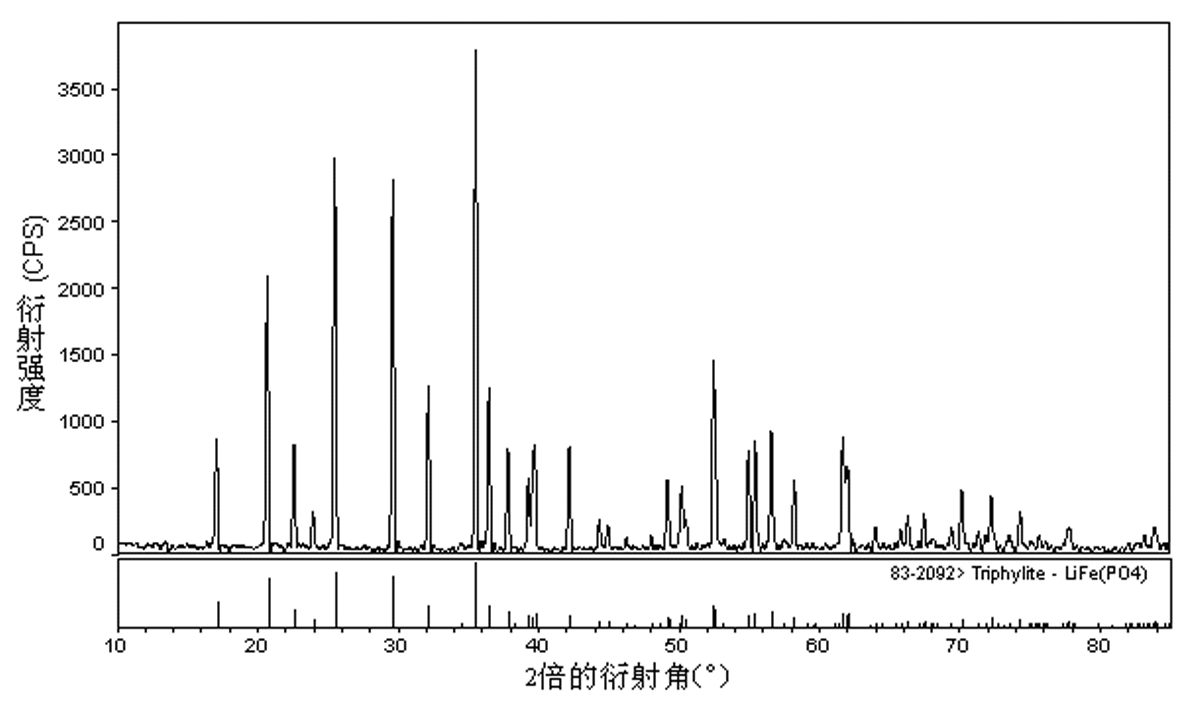
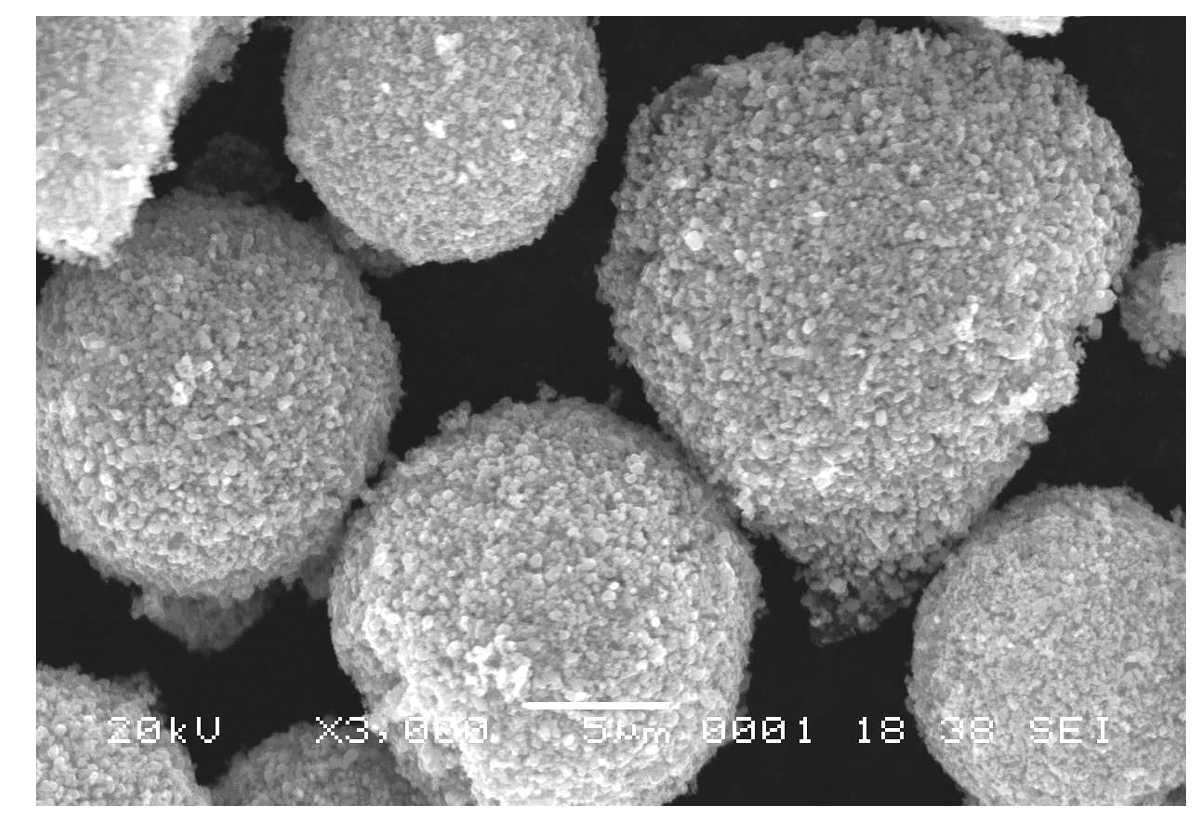
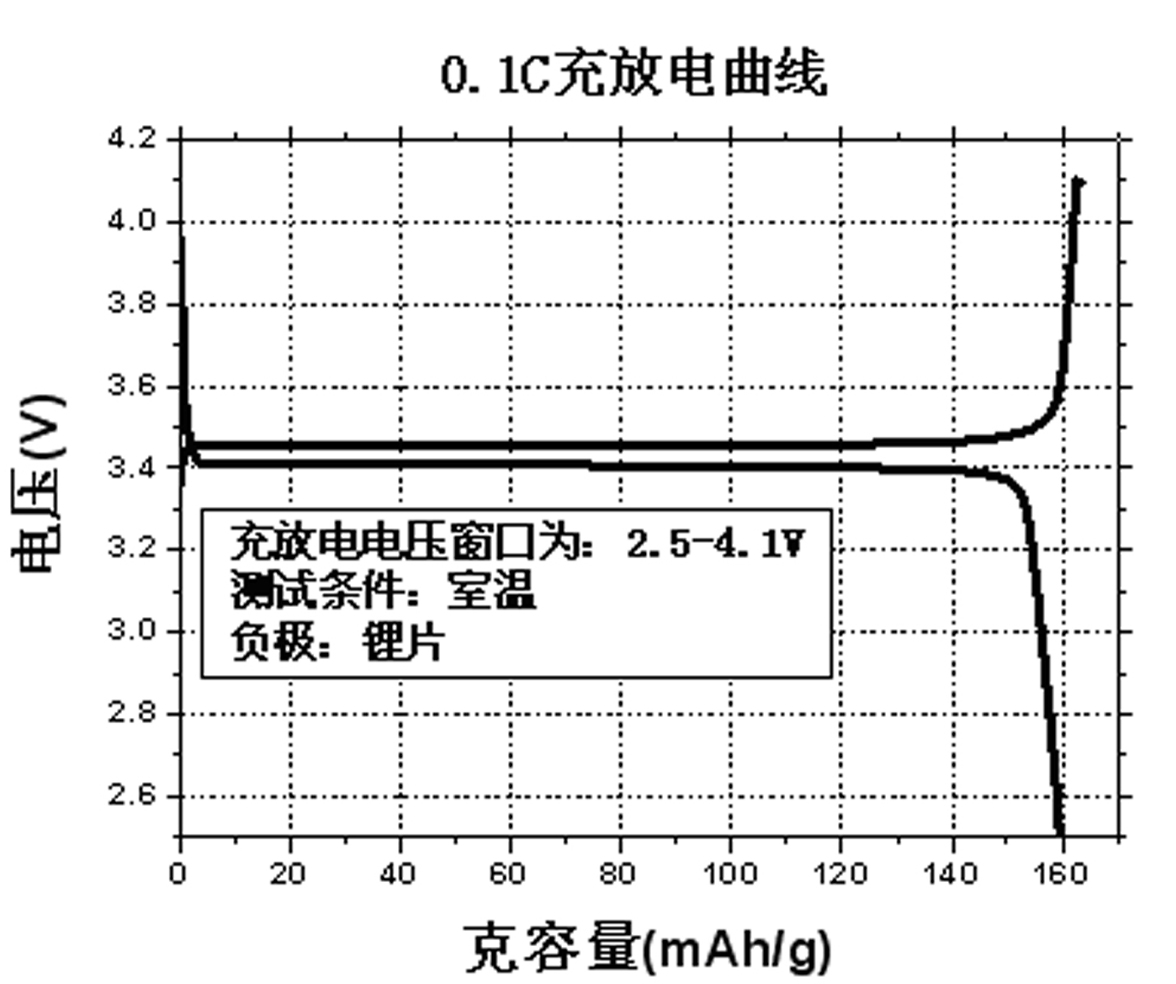
Method for preparing high-density lithium ferric phosphate
A lithium iron phosphate, high-density technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as incomplete coating, reduced product capacity, low conductivity, etc., to improve bulk density and processability Excellent, reduce the effect of contact conductance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 369.45g of lithium carbonate, 1780.96g of ferrous oxalate, 1150.26g of ammonium dihydrogen phosphate and 14.84g of magnesium oxalate into 5000g of acetone, first ball mill in a sand mill with 5mm zirconia balls for 3 hours, and then add acetone solvent 10000g, turn it into a sand mill with a zirconia ball size of 0.5mm for 6 hours, and dry the slurry for the first time in a blast drying oven at 80°C. The first dried material was put into a tube furnace, filled with nitrogen protection, and pretreated at 400°C for 3 hours. To the pretreated product, pass through jaw crusher, double roller machine and jet mill, add the lithium dihydrogen phosphate (molecular formula LiH2) of 3wt% of predecomposed product weight 2 PO 4 ), added together in 2000g of deionized water, and milled for 1 hour with a sand mill with 5mm zirconia balls, and the slurry was dried for the second time in a blast drying oven at 100°C. The secondary dried material is put into a tube furnace, filled...
Embodiment 2
[0028] 659.85g lithium acetate (molecular formula CH 3 COOLi), 1780.96g ferrous oxalate (molecular formula FeC 2 o 4 2H 2 O), 1150.26g ammonium dihydrogen phosphate (molecular formula NH 4 h 2 PO 4 ) and 7.99g of titanium dioxide (molecular formula TiO 2 ) was added to 6000g of ethylene glycol, first ball milled in a sand mill with 5mm zirconia balls for 6 hours, then supplemented with 10000g of ethylene glycol solvent, and then milled with a 0.3mm zirconia ball for 3 hours, The slurry was dried for the first time in a blast oven at 80°C. The first dried material was put into a tube furnace, filled with nitrogen protection, and pretreated at 450°C for 6 hours. To the pretreated product, pass through the jaw crusher, the roller mill and the jet mill, add the lithium dihydrogen phosphate (molecular formula LiH2) of 5wt% of the predecomposed product weight 2 PO 4 ), added together in 2500g of deionized water, and milled for 1 hour with a sand mill with 5mm zirconia balls...
Embodiment 3
[0032] 419.64g lithium hydroxide (molecular formula LiOH·H 2 O), 1780.96g ferrous oxalate (molecular formula FeC 2 o 4 2H 2 O), 1320.56g diammonium hydrogen phosphate (molecular formula (NH 4 )2HPO 4 ) and 13.29g niobium pentoxide (molecular formula Nb 2 o 5 ) was added to 6000g of acetone, first milled in a sand mill with 5mm zirconia balls for 4 hours, then supplemented with 10000g of acetone solvent, and then milled with a sand mill with 0.5mm zirconia balls for 2 hours, the slurry was ground at 80 Do the first drying in a forced air drying oven at ℃. Put the dried material for the first time into a tube furnace, fill it with nitrogen protection, and pretreat it at 350°C for 10h. To the pretreated product, pass through jaw crusher, double roller machine and jet mill, add the lithium dihydrogen phosphate (molecular formula LiH2) of 2wt% of predecomposed product weight 2 PO 4 ), added together in 2500g of deionized water, milled for 1 hour with a sand mill with 5mm z...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com