Supported polypropylene imine material and preparation method and application thereof
A polypropylene imine and application technology, applied in the field of supported polypropylene imine material and its preparation, can solve the problems of limiting practical application and high synthesis cost, and achieve the advantages of large-scale production, high selectivity and large adsorption capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Polypropylene imine synthesis
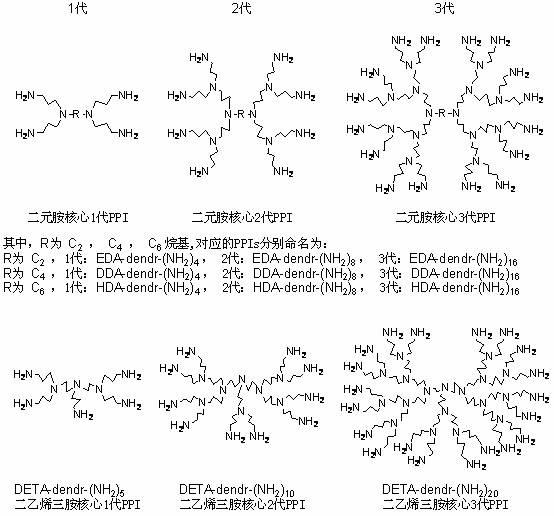
[0027] EDA-dendr-(NH 2 ) 4 The synthesis is as follows:
[0028] Add 60g (1mol) of ethylenediamine and 120g of distilled water to a 500ml three-neck flask equipped with a reflux condenser, a thermometer, a constant pressure dropping funnel and a magnetic stirrer. The temperature of the reaction system was raised to 25° C., and 265 g (5 mol) of acrylonitrile was slowly added dropwise within two hours using a constant pressure dropping funnel, and the temperature was raised to 40° C. for 1 hour to react. Then, slowly raise the temperature to 80°C, azeotropically reflux, and react for 20 hours. Finally, after cooling down to room temperature, water and excess acrylonitrile were removed by rotary evaporation, and the remaining crude product was a yellow viscous liquid. The above crude product was dissolved in 3×800ml of hot ethanol, recrystallized to obtain white flaky crystals, and dried in vacuum at 40°C for 5 hours to obtain an...
Embodiment 2-6
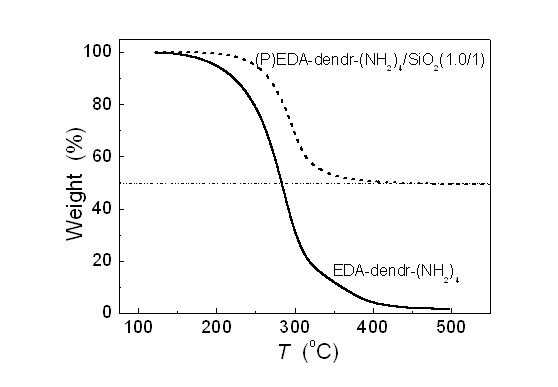
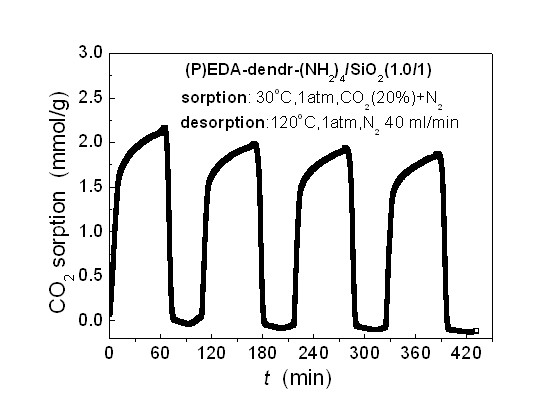
[0032] Take 5g EDA-dendr-(NH 2 ) 4 , dissolved in 80 ml absolute ethanol. Add 10 g of porous silica gel to the above solution, stir for 1 hour to fully impregnate; slowly evaporate the solvent at 50° C., and continue vacuum drying at 80° C. for 48 hours to obtain a white powder product. The resulting product is denoted as (P)EDA-dendr-(NH 2 ) 4 / SiO 2 (0.5 / 1). Among them, P represents the physical load by impregnation method, EDA-dendr-(NH 2 ) 4 and SiO 2 respectively represent the corresponding polypropylene imine and porous carrier material, 0.5 / 1 represents EDA-dendr-(NH 2 ) 4 with SiO 2 The mass ratio of the two.
[0033] Similarly, other polypropyleneimine supported materials were prepared, see Table 1.
[0034] Table 1 Load conditions of Examples 2-6
[0035] Example
Embodiment 7
[0037] Into a dry 250ml four-necked flask equipped with a thermometer, a constant pressure dropping funnel, a magnetic stirrer and a nitrogen conduit, add 28.8g of porous silica gel powder, 100mL of anhydrous toluene, nitrogen protection, raise the temperature to 80°C, and drop Slowly add 0.1mol γ-glycidyloxypropyltrimethoxysilane dropwise to the liquid funnel within 1 hour, and react for 8 hours. The reaction mixture was cooled to room temperature, and solvents such as toluene were removed by rotary evaporation to obtain a powdery product. Vacuum drying at 80°C for 48 hours to obtain glycidyl-containing silica gel powder.
[0038] Add 0.1mol EDA-dendr-(NH 2 ) 4 , 100mL absolute ethanol, under nitrogen protection, then add the above-mentioned pretreated porous silica gel powder, and react at room temperature for 4h. Solvents such as ethanol were removed by rotary evaporation to obtain a powdery product. Vacuum drying at 80°C for 48 hours gave the final product as a white p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com