Method for preparing nano nickel by taking nano spherical polyelectrolyte brush as reactor and application of nano nickel
A polyelectrolyte brush and polyelectrolyte technology, applied in chemical instruments and methods, nanotechnology, nanotechnology, etc., can solve problems such as easy agglomeration, difficulty in controlling the size and distribution of nano-nickel, and achieve good catalytic activity and excellent dispersion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
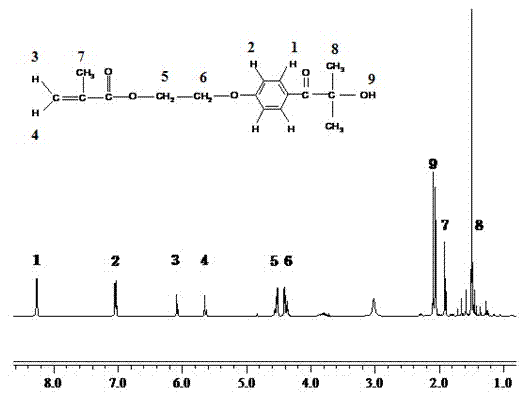
[0033] Preparation of Photoinitiator HMEM by Schotten-Baumann Reaction
[0034] Pour 30.0g of photoinitiator 2-hydroxy-4'-hydroxyethoxy-2-methylphenyl ethyl ketone (HMP) into a 500ml single-necked flask, add 150ml of acetone, stir until HMP is completely dissolved, then add 10g of newly purified pyridine, continue to stir at low temperature, then dissolve 13.6g of methacryloyl chloride (MC) in 50ml of acetone, and slowly add it dropwise into the reaction flask, after the dropwise addition, react at room temperature for 12 hours to obtain Orange liquid, the beige precipitate at the bottom is removed by filtration, and the chromatographic separation method is used to obtain pure HMEM photoinitiator, and its proton nuclear magnetic resonance spectrogram is as follows image 3 shown.
Embodiment 2
[0036] Nanospherical Polyelectrolyte Brushes Prepared by Photoemulsion Polymerization
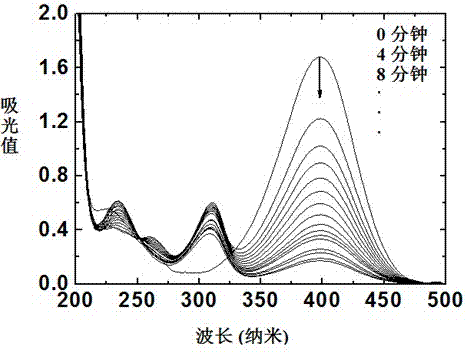
[0037]Put 230 ml of deionized water, 0.24 g of sodium dodecylsulfonate (or sodium dodecylbenzenesulfonate), and 0.74 g of potassium persulfate (or hydrogen peroxide) into a 500 ml three-neck flask. After fully dissolved, add 10.0 g of styrene (or methyl methacrylate). Pump nitrogen 3 to 5 times, and control the speed at 300 rpm. After the temperature rose to 35 °C, 0.15 g of sodium bisulfite was dissolved in 20 ml of deionized water, and quickly dropped into the three-necked flask. During the reaction, keep the temperature and rotating speed constant, and after 2 hours, the polymerization reaction reaches the final stage. At this time, 1.0 gram of the photoinitiator HMEM obtained in Example 1 is slowly added dropwise to the reaction system, and the rate of addition is controlled at 7 seconds. / drop; continue to react for 3 hours after the drop is completed; the polymer microemulsion ball...
Embodiment 3
[0039] Preparation of nanospherical polyelectrolyte brushes adsorbed with nickel ions
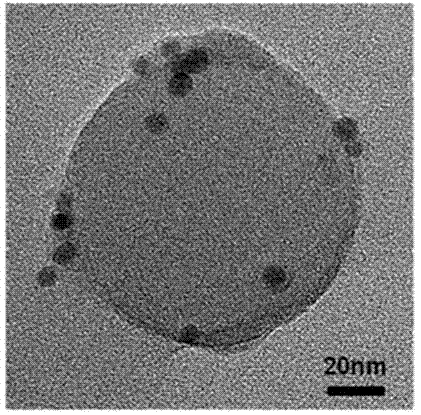
[0040] Get 50 milliliters of the spherical polyelectrolyte brush emulsion that embodiment 2 obtains, add 0.024 gram of sodium hydroxide solids, after fully stirring for 10 hours, place this emulsion in an ultrafiltration device, adopt 3 liters of concentration to be 0.001 mol / liter of nickel chloride The aqueous solution (nickel chloride hexahydrate or nickel acetate tetrahydrate can be used) is subjected to ultrafiltration, so that nickel ions and sodium ions undergo ion exchange, enter the interior of the nano-spherical polyelectrolyte brush, and then use 3 liters of deionized water for ultrafiltration washing to remove free nickel ions, and the nano-spherical polyelectrolyte brushes adsorbed with nickel ions are obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| hydrodynamic diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com