High expression of tenebrio molitor antibacterial peptide TmAMP3m in escherichia coli and application of TmAMP3m
An antibacterial peptide, Tenebrio molitor technology, applied in the application, antibacterial drugs, medical preparations containing active ingredients, etc., can solve the problems of failure to realize industrialized production, low content of natural antibacterial peptides, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: High expression of recombinant mutant Tenebrio molitor antimicrobial peptide TmAMP3m in Escherichia coli
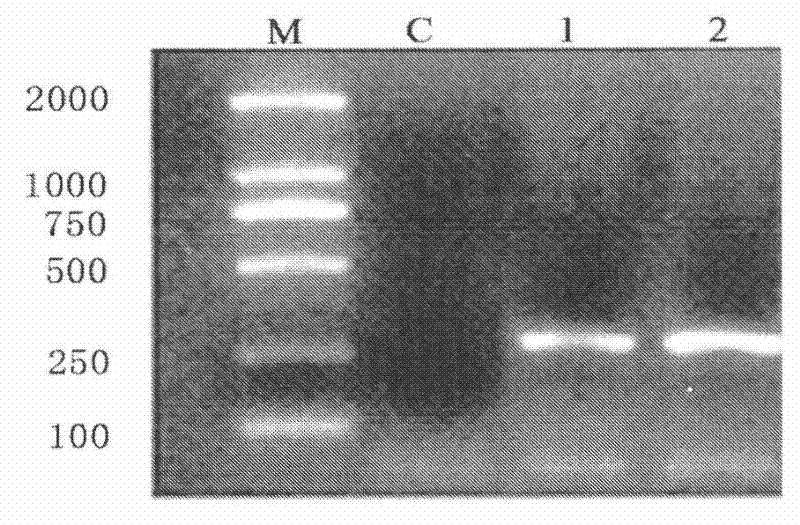
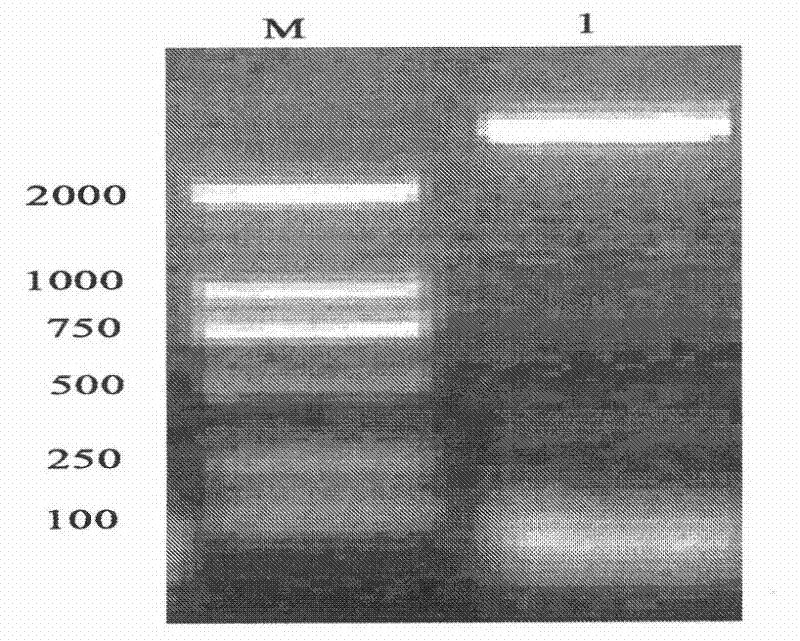
[0075] See attached Figure 1-4 :
[0076] (1) Design specific primers using the antimicrobial peptide sequence of Tenebrio molitor (Accession No. TMU21482) published by GenBank:
[0077] Upstream primer TF1: 5′-ATGAAAACATTCGTGATTTGCTTGATTCTGG-3′,
[0078] Downstream primer TR1: 5′-TTAATGA CCATGTGTCTTGTACCCTCCTTGG-3′;
[0079] (2) The antimicrobial peptide gene tenecin3 was cloned from Tenebrio molitor by RT-PCR
[0080] Before the operation, the laboratory bench was irradiated with ultraviolet light for 30 minutes, and a 5-year-old Tenebrio molitor larva was taken and ground in an RNase-free mortar with liquid nitrogen. The powder was transferred into an Eppendorf tube containing 1 mL of Trizol reagent, and the cap was tightly shaken. 15s, place at room temperature at 15-30°C for 3-5min, centrifuge at 12000r / min at 4°C for 15min, carefully transfer ...
Embodiment 2
[0107] Example 2: Prokaryotic expression of recombinant plasmid pET30a-TmAMP3m
[0108] Mix the extracted plasmid DNA DH5α / pET30a-TmAMP3m with 35 μL of competent cells E.coliBL21, place in an ice bath for 30 minutes, then heat shock in a water bath at 42°C for 45 seconds, and quickly remove from the ice bath for 3 minutes (do not shake the EP tube). Add 800 μL of fresh LB culture medium without antibiotics to each tube, mix well, and incubate at 37°C for 1 h with gentle shaking. 4°C, 3000rpm, centrifuge for 1min, discard 600μL supernatant, gently pipette and mix the remaining bacterial solution, spread evenly on the prepared solid LB medium (containing kanamycin), and culture overnight at 37°C. The next day, pick a single clone and culture it in 6mL LB medium (containing kanamycin) for 12-16h, then transfer it to fresh medium (containing Kana) at a volume ratio of 1% to 2%, at 37°C, 220r Shake the bacterial solution at 0.4-0.6 per minute until the OD600 is 0.4-0.6, and pipett...
Embodiment 3
[0109] Example 3: Antibacterial Activity Detection Method of Recombinant Mutant Tenebrio Molitor Antimicrobial Peptide TmAMP3m
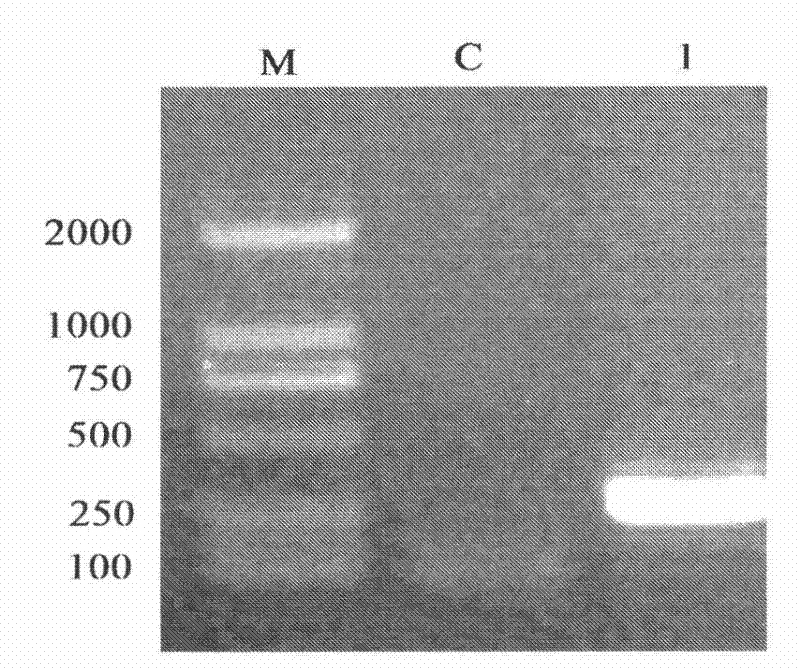
[0110] First, 3 μl of the antimicrobial peptide obtained by expression was taken out. Take Staphylococcus aureus as the experimental strain, culture in LB liquid medium until OD600=0.5, refer to the agar hole diffusion method, take 10μl of bacterial suspension and 3ml of solid medium (containing 0.8% agar) and mix at 45°C , and spread it in a sterile petri dish with a diameter of 6cm, and store it at 4°C after solidification; when in use, make a number of small holes with a diameter of 2mm in the dish, and drop 3μl of the product to be tested into the holes, and store at 37°C Cultivate under conditions for 12-16h. Measure the size of the zone of inhibition. According to the size of the inhibition zone to determine the activity of antimicrobial peptides, see the attached Figure 7 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com