Gradient active scaffold material for osteochondral repair and its preparation method and application
A scaffold material, osteochondral technology, applied in medical science, prosthesis and other directions, can solve the problems of poor repair ability, low metabolic rate physiological characteristics, disease transmission and other problems, and achieve the effect of simple preparation method, firm adhesion and adhesion promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
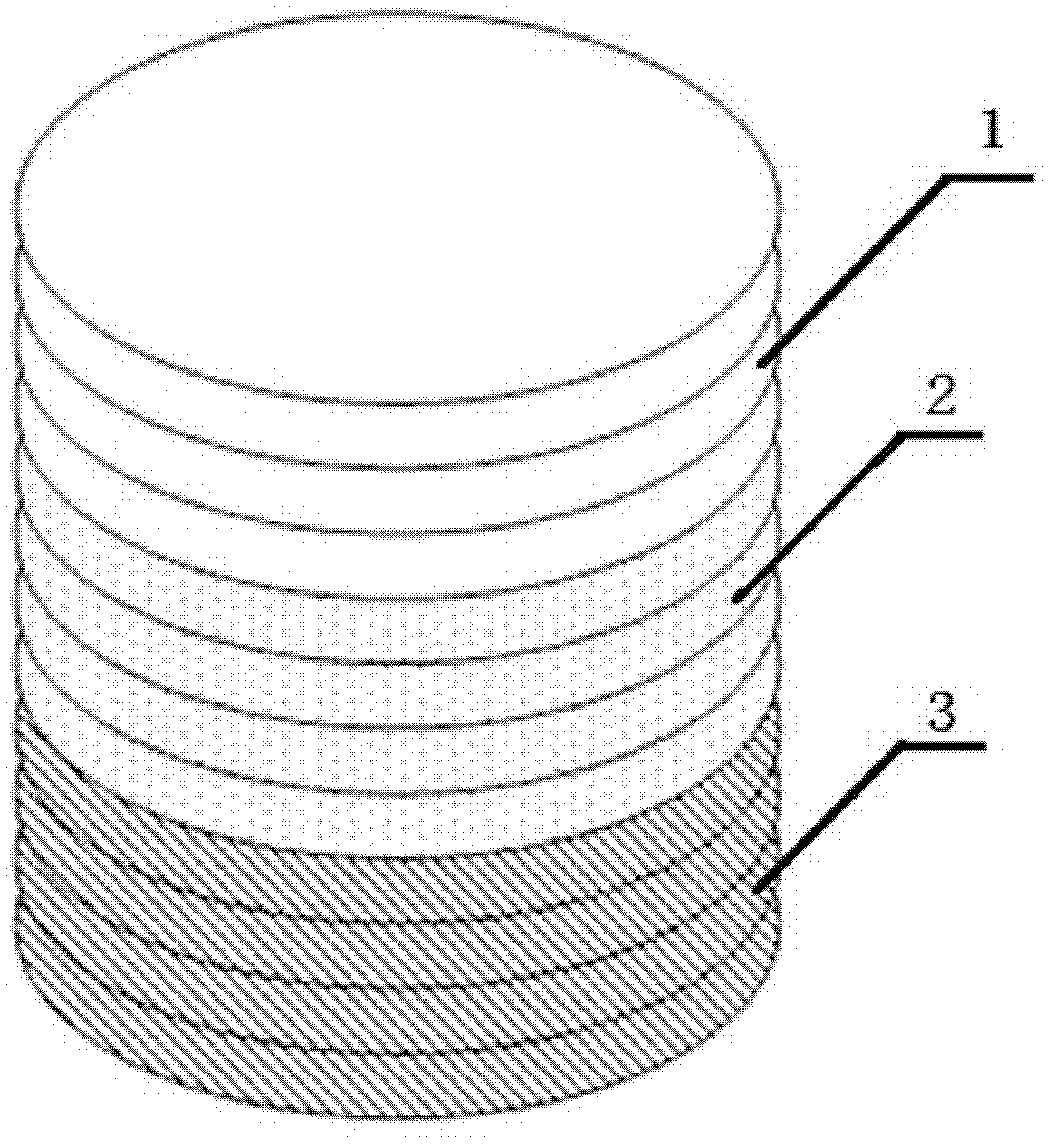
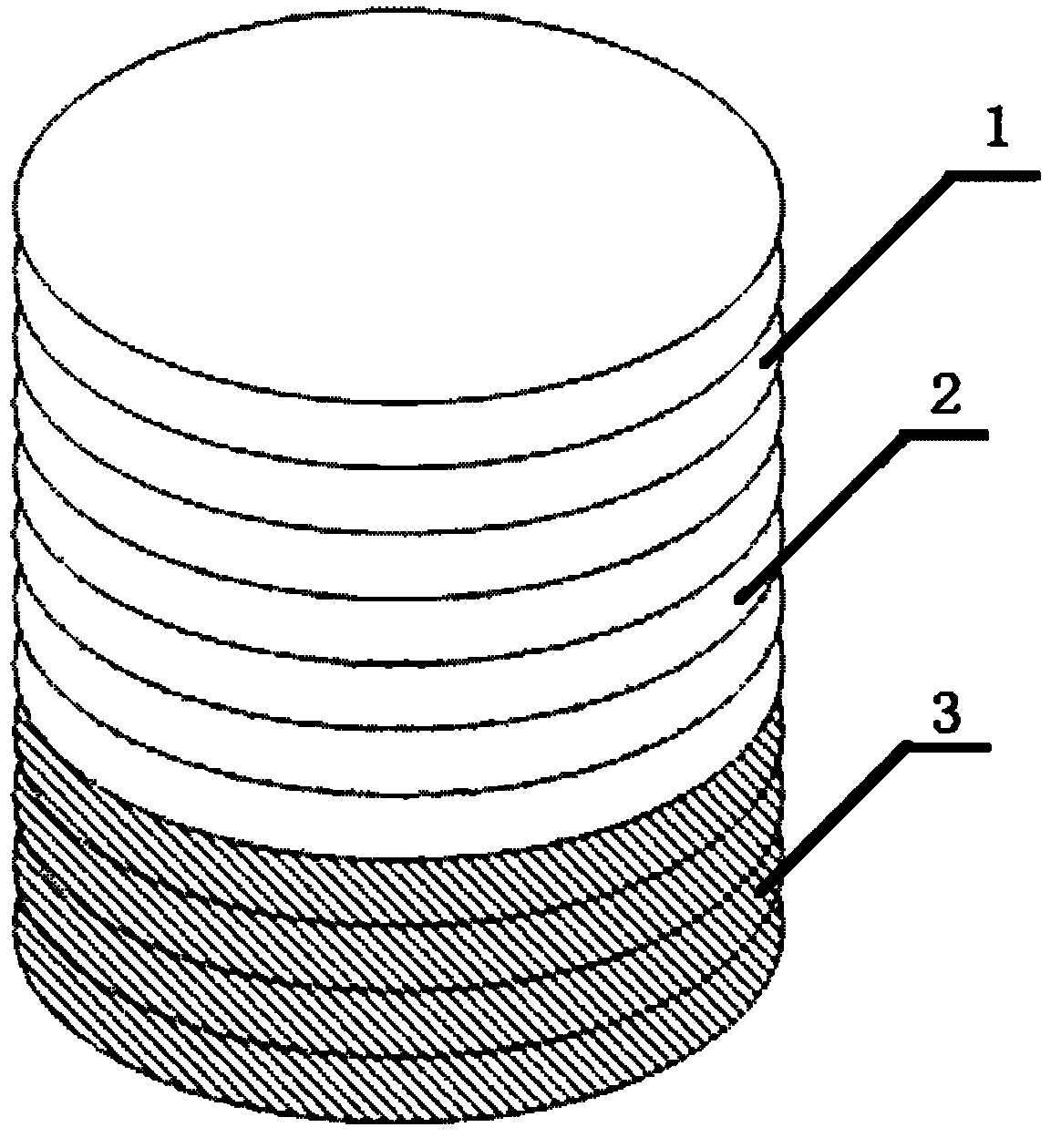
[0052] (1) Type I collagen, calcium nitrate tetrahydrate, and diammonium hydrogen phosphate are used as raw materials, and ammonia water is used to adjust PH=8.0, and HA / Col powder is prepared by coprecipitation method. In the prepared HA / Col-I composite powder, Type I collagen content is 35% and hydroxyapatite content is 65%;
[0053] (2) Use the EDC / NHS-Heparin method to construct a controlled release system for biologically active substances, and soak the HA / Col-I composite powder and the previously prepared type II collagen powder in 0.05mol / LMES buffer (pH 5.6) 30min for standby, dissolve each 2.5g of heparin in 300ml of 0.05mol / L MES buffer containing 2gEDC and 1.5gNHS for activation for 10min, put the spare collagen into the MES containing heparin and EDC / NHS according to the weight ratio of collagen to heparin 1:2 In the buffer solution, gently shake the reaction at a constant temperature of 37°C for 4h; then place the modified HA / Col-I composite powder and type II col...
Embodiment 2
[0057] (1) With type I collagen, calcium nitrate tetrahydrate, and diammonium hydrogen phosphate as raw materials, ammonia water is used to adjust the pH value to 8.0, and HA / Col powder is prepared by co-precipitation method. In the prepared HA / Col-I composite powder , the content of type I collagen is 20%, and the content of hydroxyapatite is 80%;
[0058] (2) Use the EDC / NHS-Heparin method to construct a controlled release system for biologically active substances, and soak the HA / Col-I composite powder and the previously prepared type II collagen powder in 0.05mol / LMES buffer (pH 5.6) 30min for standby, dissolve every 3g of heparin in 200ml of 0.05mol / L MES buffer containing 1gEDC and 2gNHS for activation for 10min, put the spare collagen into the MES buffer containing heparin and EDC / NHS according to the weight ratio of collagen to heparin 1:3 Gently shake and react for 4 hours at a constant temperature of 37°C; then place the modified HA / Col-I composite powder and type II...
Embodiment 3
[0062] (1) With type I collagen, calcium nitrate tetrahydrate, and diammonium hydrogen phosphate as raw materials, ammonia water is used to adjust the pH value to 8.0, and HA / Col powder is prepared by co-precipitation method. In the prepared HA / Col-I composite powder , the content of type I collagen is 50%, and the content of hydroxyapatite is 50%;
[0063] (2) Use the EDC / NHS-Heparin method to construct a controlled release system for biologically active substances, and soak the HA / Col-I composite powder and the previously prepared type II collagen powder in 0.05mol / LMES buffer (pH 5.6) 30min for standby, dissolve each 1g of heparin in 500ml of 0.05mol / L MES buffer containing 3gEDC and 0.5gNHS for activation for 10min, put the spare collagen into the MES buffer containing heparin and EDC / NHS according to the weight ratio of collagen to heparin 1:1 solution, and gently shaken at a constant temperature of 37°C for 4 hours; then the modified HA / Col-I composite powder and type II...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com