Preparation method of barium carbonate powder
A technology of barium carbonate and powder, which is applied in the direction of calcium carbonate/strontium/barium, bulk chemical production, nanotechnology, etc., and can solve the problem of difficult large-scale industrial production of barium carbonate, easy bonding or agglomeration of particles, poor particle uniformity, etc. problems, to achieve the effect of simple and easy-to-operate preparation method, good dispersibility, and little solvent pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
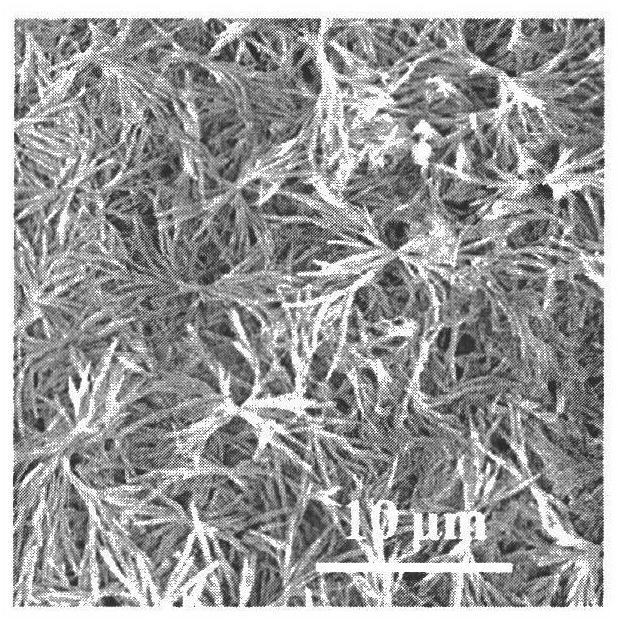
[0032] All glass instruments used in the experiment were pretreated as follows: firstly, they were sonicated in ethanol for 30 minutes, and then they were taken out and rinsed with deionized water. Put it into the mixed solvent of concentrated nitric acid / hydrogen peroxide / water according to the volume ratio of 1:1:1 and soak for 2 days, take it out, wash it with acetone, and dry it at room temperature. Prepare 0.1 g / L sodium carboxymethyl cellulose (molecular weight is 7×10 5 g / mol) aqueous solution 50 milliliters, then add a certain amount of barium hydroxide and be mixed with the barium hydroxide solution of 0.025 mol / liter, adjust the pH of solution to 8. Transfer the beaker containing the solution to a stainless steel autoclave, seal and evacuate until the vacuum degree is less than 10Pa. The temperature of the reaction oil bath was controlled to be 35° C., and carbon dioxide gas was slowly charged to make the pressure in the kettle be 4.5 MPa, and the reaction was maint...
Embodiment 2
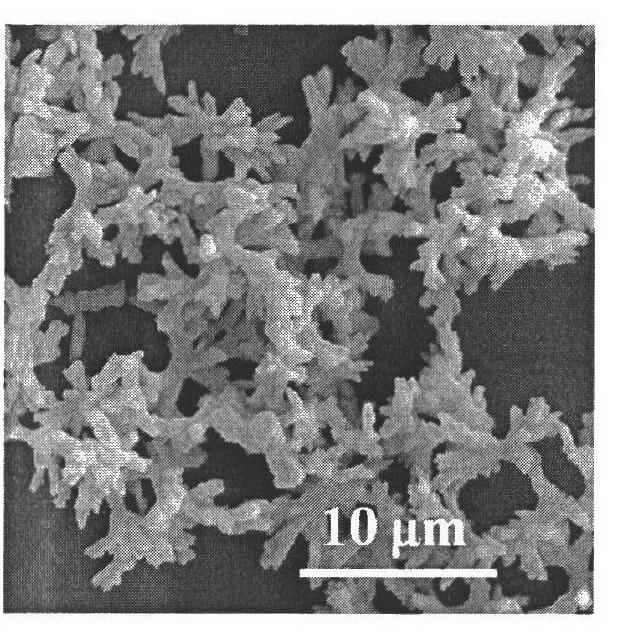
[0034] The glass instrument processing method is the same as in Example 1. The method of operation of the experiment is also basically the same as in Example 1, except that sodium carboxymethylcellulose (molecular weight is 9 × 10 4 (gram / mole) aqueous solution concentration is 2 grams / liter, and the concentration of barium hydroxide solution is 0.03 mole / liter, and the pH of solution is 8.5, and reaction oil bath temperature is 50 ℃, and pressure in still is 6.5 MPa, and reaction time is 60 minute. Finally, the drying method for the obtained barium carbonate powder is as follows: drying at 40° C. for 10 hours under a vacuum degree of about 10 Pa. From figure 2It can be seen that the barium carbonate particles obtained in this embodiment are still a symmetrical dendritic structure bundled from the middle, but compared to the structure obtained in Example 1, the overall structure of the barium carbonate particles obtained in this embodiment The length is between 4-8 microns...
Embodiment 3
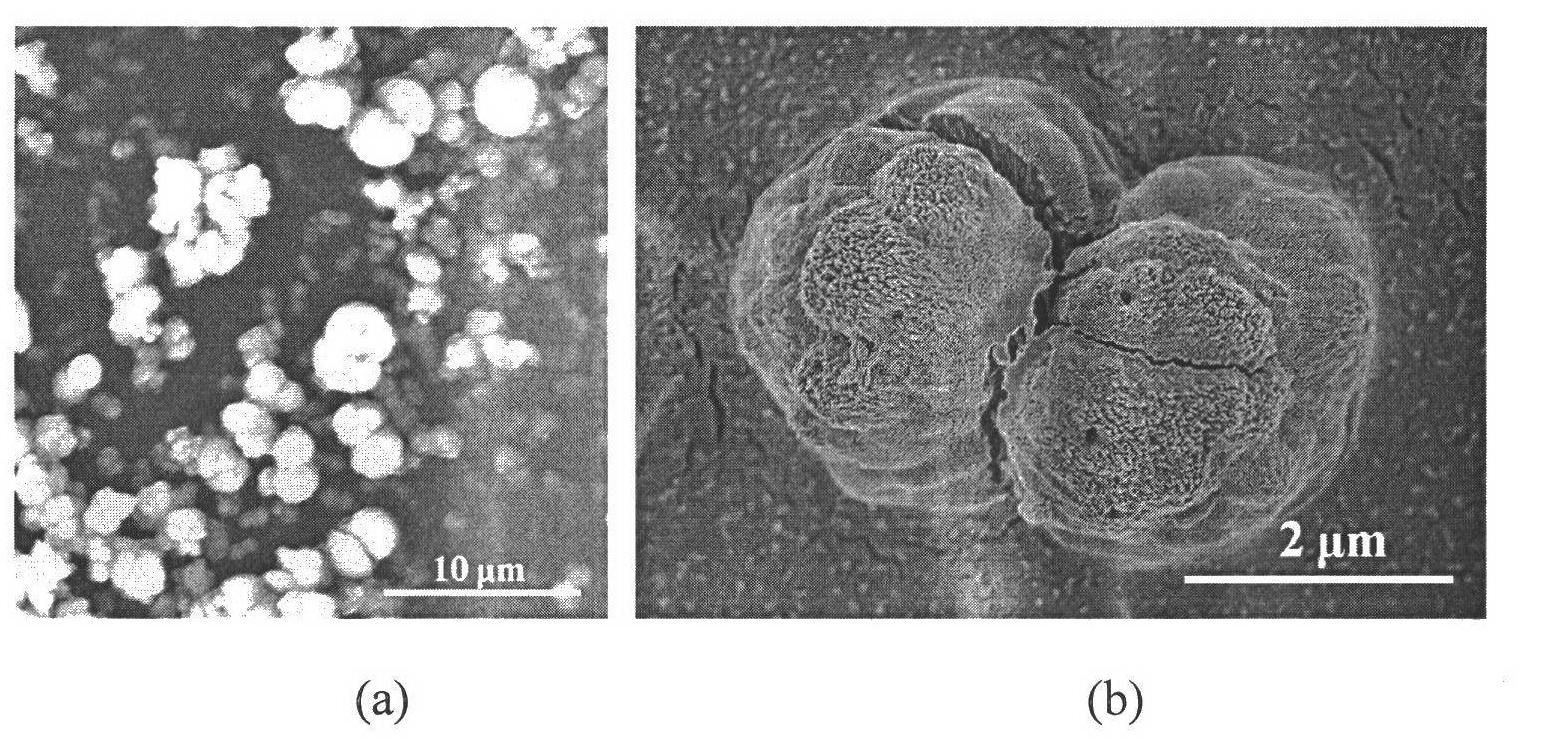
[0036] The glass instrument processing method is the same as in Example 1. The method of operation of the experiment is also basically the same as in Example 1, except that sodium carboxymethylcellulose (molecular weight is 9 × 10 4 (gram / mole) aqueous solution concentration is 10 grams / liter, and the concentration of barium hydroxide solution is 0.035 mole / liter, and the pH of solution is 7.5, and reaction oil bath temperature is 25 ℃, and still internal pressure is 3.5 MPa, reaction time 30 minute. Finally, the drying method for the obtained barium carbonate powder is as follows: drying at 25° C. for 24 hours under a vacuum degree of about 100 Pa. From image 3 It can be seen from the figure that the barium carbonate particles obtained in this example are almost all double-sphere structures, the diameter of the balls is between 500 nanometers and 1 micron, and the overall length is between 1-4 microns. It can be seen from an enlarged picture of double-sphere particles tha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com