Method for preparing high purity rare earth metal and its apparatus
A rare-earth metal, high-purity technology, applied in the direction of improving process efficiency, can solve the problems of small removal effect, low production efficiency, high content of metal gas impurities and non-rare earth metal impurities, so as to reduce the content of gas impurities and improve utilization The effect of improving the efficiency and improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
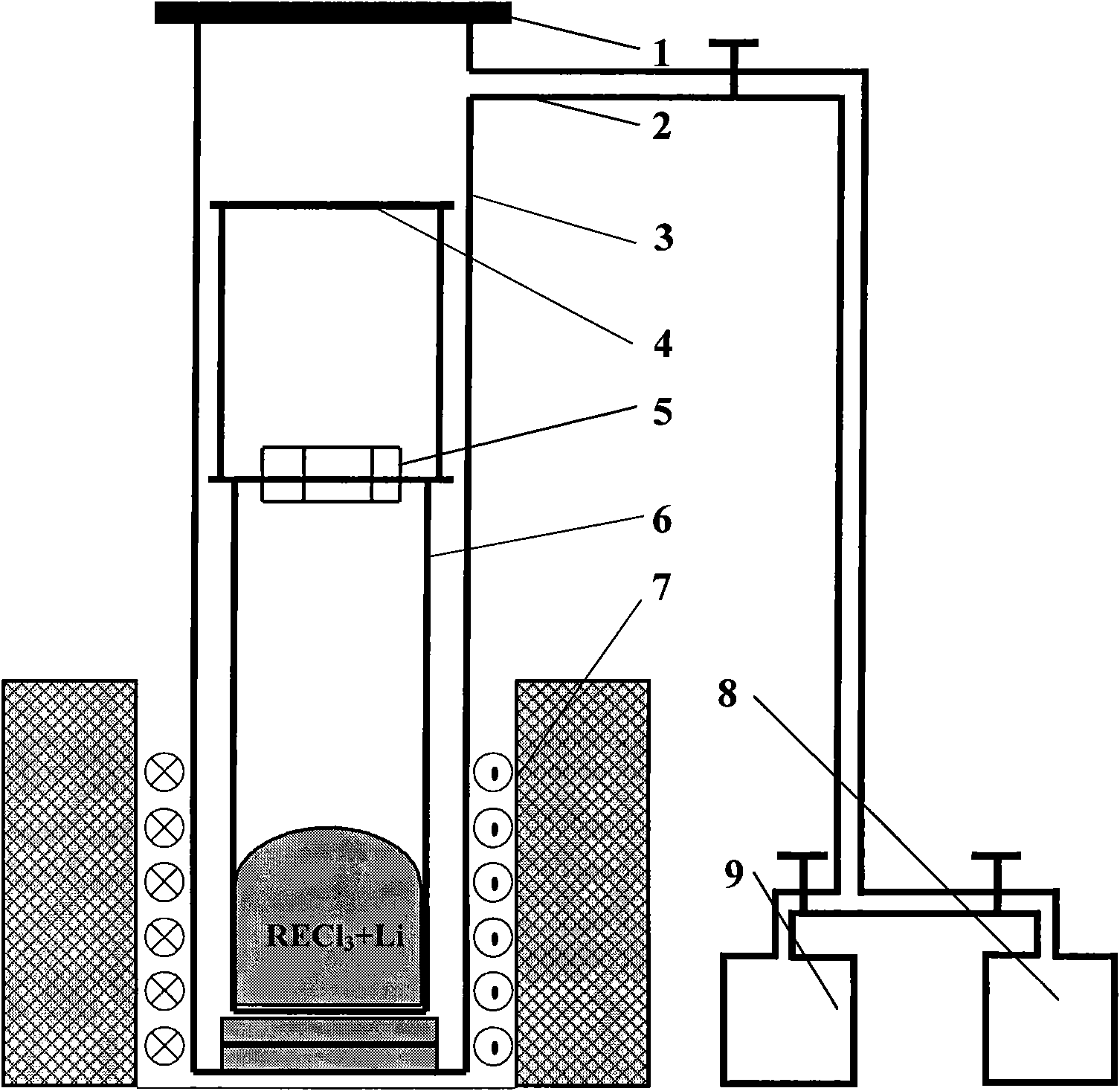
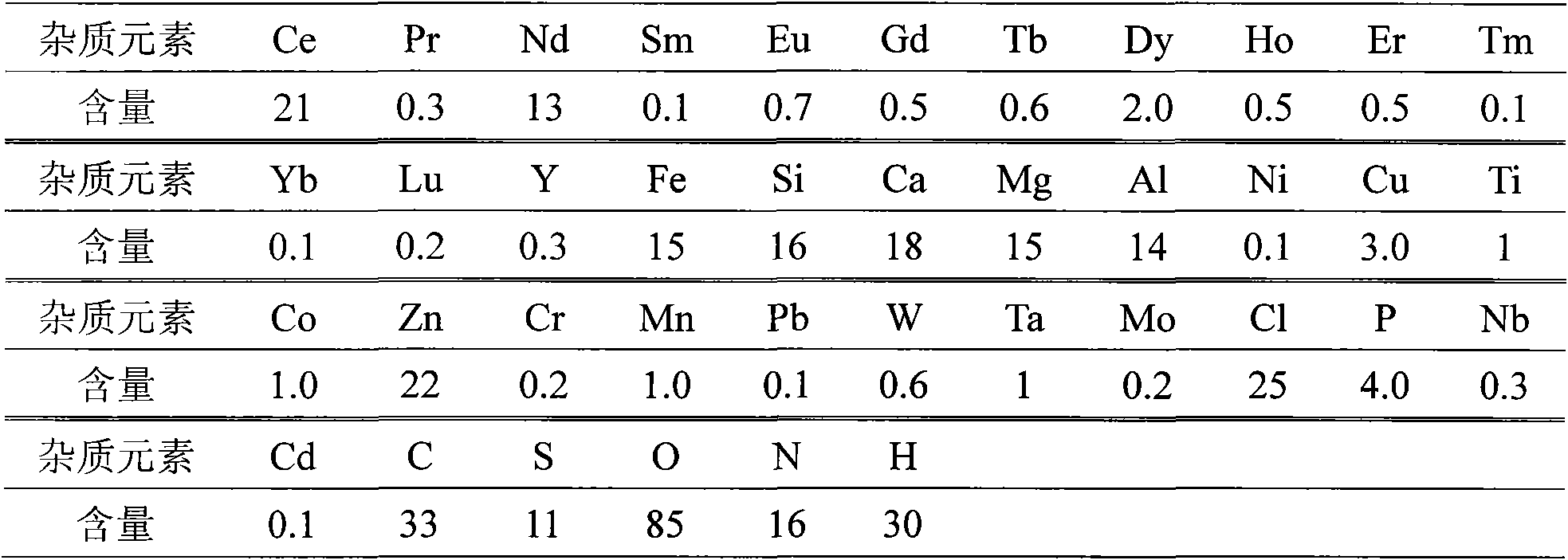
[0031] Attached Figure 1 The equipment and device shown, add 500g of anhydrous lanthanum chloride with a purity of 99.9wt.% and 45g of lithium metal with a purity of 99.9wt.% to a titanium crucible made of titanium with a purity of 99.5wt.%, in a 99.999% argon atmosphere (>0.1MPa), raise the temperature to 950°C (the pressure of the reaction system is kept below 0.5MPa during the heating process), and keep it warm for 1 hour, then pump the system down to below 0.1Pa for distillation and removal of impurities, and keep it warm for 3 hours at 1100°C After finishing the distillation, refill with argon and cool to room temperature. Reduction obtained 263.4g of metal lanthanum, the yield was 93%, and the absolute purity of lanthanum was 99.957%. The analysis results are as follows:
[0032] Table 1 Metal Lanthanum Composition Analysis Unit: ppm (wt.)
[0033]
Embodiment 2
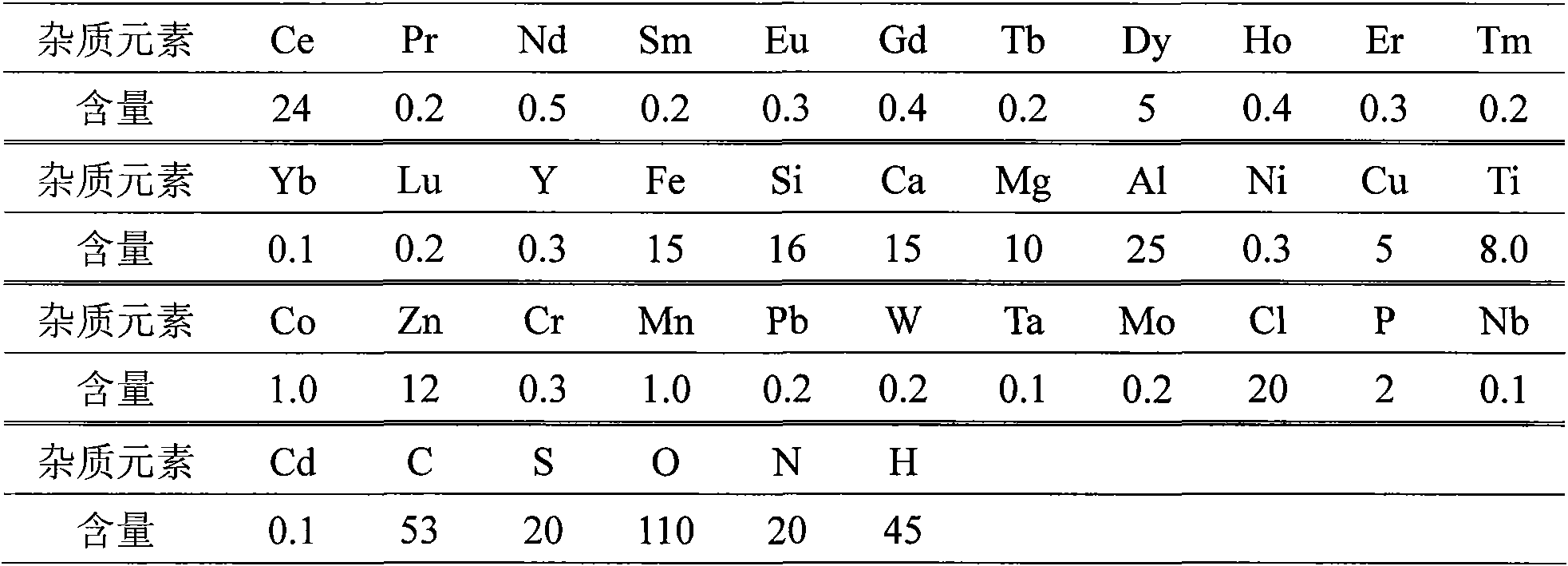
[0035] Attached Figure 1 For the equipment and device shown, add 500g of anhydrous lanthanum chloride with a purity of 99.9wt.% and 48g of lithium metal with a purity of 99.9wt.% to a tantalum crucible made of a tantalum material with a purity of 99.9wt.%, in a 99.999% argon atmosphere (>0.1MPa), raise the temperature to 950°C (the pressure of the reaction system is kept below 0.5MPa during the heating process), and keep it warm for 1 hour, then pump the system down to below 0.1Pa for distillation and removal of impurities, and keep it warm for 3 hours at 1100°C After finishing the distillation, refill with argon and cool to room temperature. The reduction obtained 265.1 g of metal lanthanum, the yield was 93.6%, and the absolute purity of lanthanum was 99.964%. The analysis results are as follows:
[0036] Table 2 Analysis of metal lanthanum components Unit: ppm (wt.)
[0037]
Embodiment 3
[0039] Attached Figure 1 The equipment and device shown, add 500g of anhydrous neodymium chloride with a purity of 99.9wt.% and 45g of lithium metal at 99.9wt.% to a titanium crucible made of titanium with a purity of 99.5wt.%, in a 99.999% argon atmosphere (>0.1MPa), raise the temperature to 1050°C (the pressure of the reaction system is kept below 0.5MPa during the heating process), and keep it warm for 1 hour, then pump the system down to below 0.1Pa for distillation and removal of impurities, and keep it warm for 3 hours at 1100°C After finishing the distillation, refill with argon and cool to room temperature. The reduction obtained 264.4g of neodymium metal, the yield was 92%, and the absolute purity of neodymium was 99.952%. The analysis results are as follows:
[0040] Table 3 Analysis of metal neodymium composition Unit: ppm (wt.)
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com