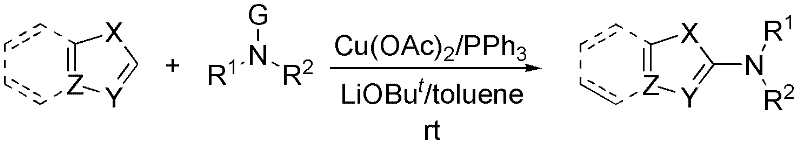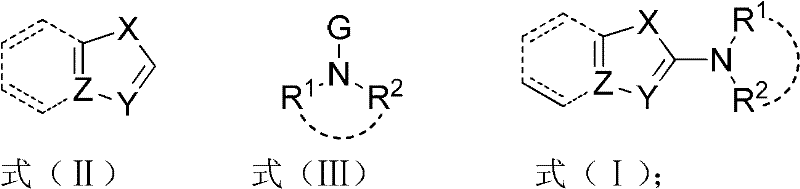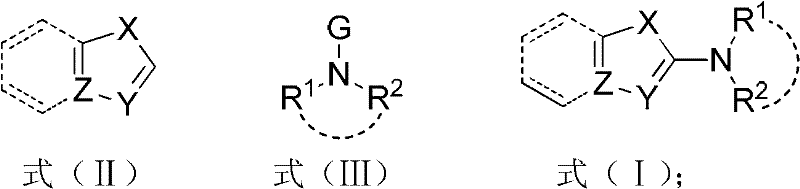Method for synthesizing 2-amino five-membered heterocyclic derivative
A compound and secondary amine technology, applied in the field of synthesizing 2-amino five-membered heterocyclic derivatives, can solve the problems of high reaction temperature, difficult to obtain raw materials, waste of amine, etc., and achieve the effect of low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, X=S among the preparation formula (I), Y=N, Z=C, R 1 , R 2 Together for (CH 2 ) 2 O(CH 2 ) 2 compound of
[0039] In a 50 ml three-neck flask equipped with magnetic stirring, add 0.1 mmol of anhydrous Cu(OAc) 2 , 0.2 mmol PPh 3 , 1.5 mmol of the morpholine nitrogen polarity inversion product R 1 R 2 NG (G=C1,R 1 , R 2 Together for (CH 2 ) 2 O(CH 2 ) 2 ) and 1.2 mmol of LiOBu t , Vacuum for N 2 Gas 2 times, add 5 ml of toluene, add 1 mmol of benzothiazole with a syringe, stir at room temperature for 5 hours; stop the reaction, wash with saturated saline 3 times, dry the organic phase with anhydrous magnesium sulfate, filter, concentrate, and use a silica gel column Chromatographic separation (silica gel 200-300 mesh; petroleum ether / ethyl acetate=10:1, v / v) yielded 217 mg (98% yield) of a white solid with a melting point of 128-130°C. The obtained product was confirmed to be the target product through structural verification.
Embodiment 2
[0040] Embodiment 2, X=S among the preparation formula (I), Y=N, Z=C, R 1 =R 2 =PhCH 2 compound of
[0041] According to the method and steps described in Example 1, with 1.5 mmoles of dibenzylamine nitrogen polarity inverting substance R 1 R 2 NG (G=C1,R 1 =R 2 =PhCH 2 ) to replace the polarity inversion of morpholine nitrogen to obtain 251 mg (yield 76%) of a white solid with a melting point of 79--81°C. The obtained product was confirmed to be the target product through structural verification.
Embodiment 3
[0042] Embodiment 3, X=S in the preparation formula (I), Y=N, Z=C, R 1 , R 2 Together for (CH 2 ) 5 compound of
[0043] According to the method and steps described in Example 1, with 1.5 millimoles of hexahydropyridine nitrogen polarity reverser R 1 R 2 NG (G=Cl, R 1 , R 2 Together for (CH 2 ) 5 ) instead of the polarity inversion of the morpholine nitrogen yielded 198 mg (yield 91%) of a white solid with a melting point of 95-96°C. The obtained product was confirmed to be the target product through structure verification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com