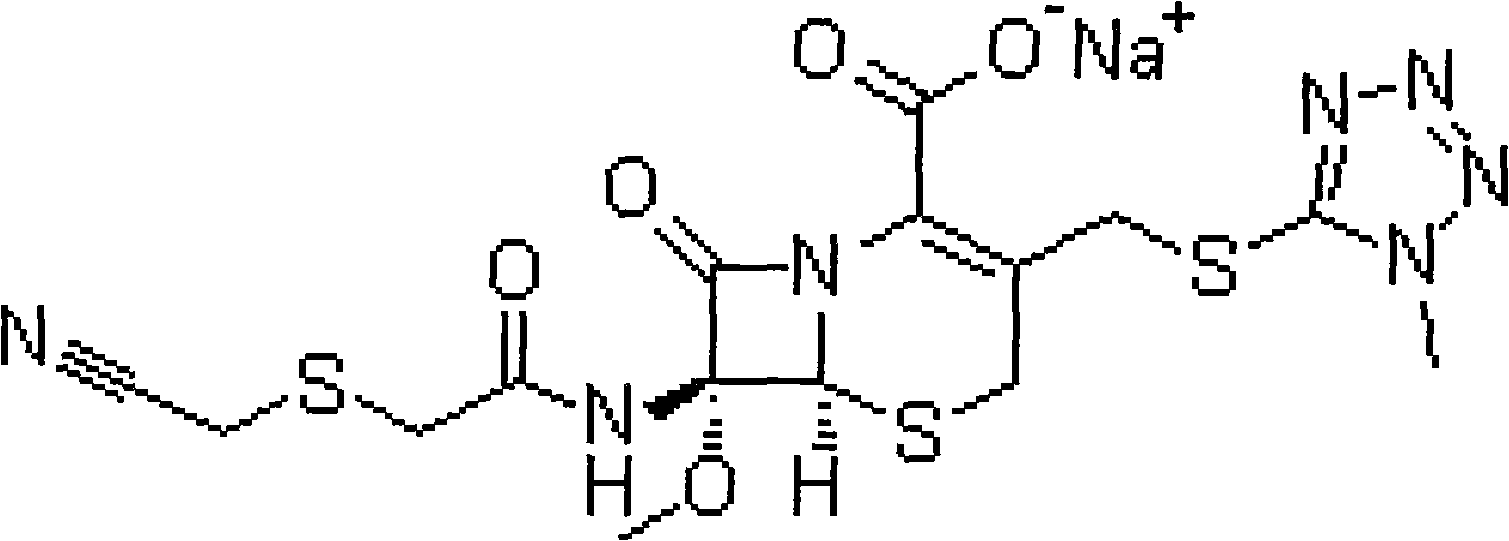
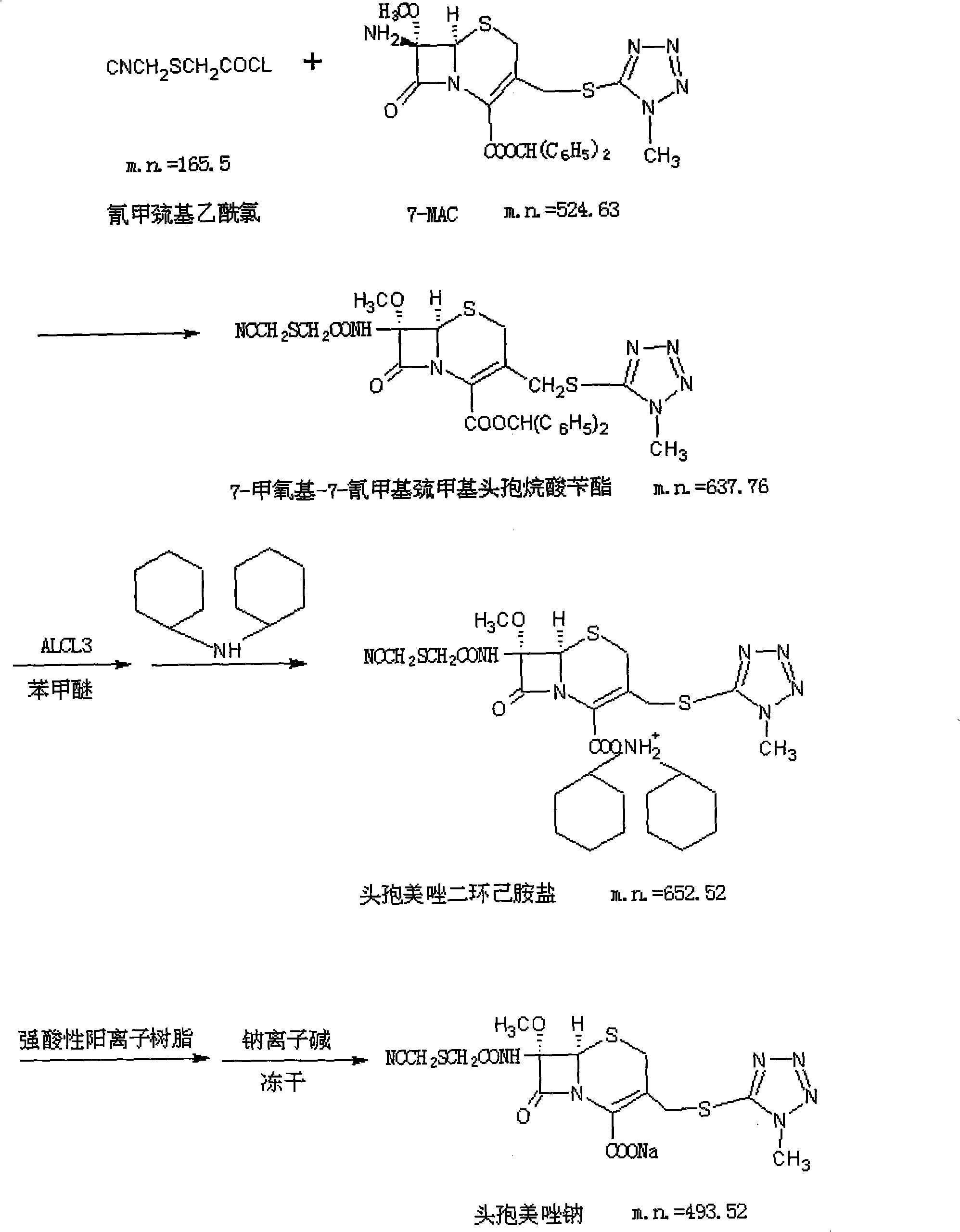
Preparation method of cefmetazole sodium
The technology of cefmetazole sodium and cefmetazole benzil is applied in the field of preparation of cephalosporins in drug synthesis technology, and can solve the problems of low production yield of cefmetazole sodium crude drug, increased production cost, increased production steps and the like, Achieve the effect of not easy to degrade, improve yield and improve product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) preparation of cefmetazole benzyl axetil:
[0049] a. Add 40g of PCL5 to 400ml of dichloromethane, cool down to -1°C, add 40g of potassium cyanomethylthioglycolate, and stir at 8°C for 1 hour to prepare a cyanomethylthioglycol chloride solution for later use.
[0050] b. Add 133g of 7-MAC (alias methoxycephalosporin) to 665ml of dichloromethane, cool down to -50°C, add 66.5g of pyridine, dropwise add the cyanomethylmercaptoacetyl chloride solution in a, react at -40°C for 1h. Add 1330ml of 3% (W / V) dilute hydrochloric acid, stir, wash and separate the phases, discard the aqueous phase, add 1330ml of 5% (W / V) NaCl to the organic phase, stir, wash and separate the phases, discard the aqueous phase, and get methazole benzyl Ester dichloromethane solution for later use.
[0051] (2) Preparation of Cefmetazolamide Salt
[0052] a. Stir and cool down 376.83g of anisole to -10°C, and slowly add an anisole solution made of 113.05g of aluminum trichloride under stirring fo...
Embodiment 2
[0057] (1) preparation of cefmetazole benzyl axetil
[0058] a. Add 60 g of PCL5 to 300 ml of dichloromethane, cool down to -3°C, add 60 g of potassium cyanomethylthioglycolate, and stir at a temperature of 12°C for 1.5 hours to prepare a cyanomethylthioglycol chloride solution for later use.
[0059] b. Add 120g of 7-MAC to 1200ml of dichloromethane, cool down to -10°C, add 120g of triethylamine, dropwise add cyanomethylmercaptoacetyl chloride solution, react at -30°C for 2h. Add 600ml of 10% (W / V) hydrochloric acid, stir, wash and separate the phases, discard the aqueous phase, add 600ml of 10% (W / V) NaCl to the organic phase, stir, wash and separate the phases, discard the aqueous phase to obtain methazole benzyl ester di Chloromethane solution for later use.
[0060] (2) Preparation of Cefmetazolamide Salt
[0061] a. Stir and cool down 211.2g of anisole to 10°C, and slowly add an anisole solution made of 105.6g of aluminum trichloride under stirring for later use.
[0...
Embodiment 3
[0066] (1) preparation of cefmetazole benzyl axetil
[0067] a. Add 84g of PCL5 to 588ml of dichloromethane, cool down to -4°C, add 56g of potassium cyanomethylthioglycolate, and stir at a temperature of 10°C for 1.2h to prepare a cyanomethylthioglycol chloride solution for later use.
[0068] b. Add 80g of 7-MAC (alias methoxycephalosporin) to 600ml of dichloromethane, cool down to -30°C, add 50g of pyridine, dropwise add cyanomethylmercaptoacetyl chloride solution, react at -35°C for 1.5h, add 560ml of 8-MAC % (W / V) hydrochloric acid stirring and washing phase separation, discarding the aqueous phase, adding 640ml of 8% (W / V) NaCl to the organic phase, stirring, washing and separating the phases, discarding the aqueous phase to obtain a methazole benzyl ester dichloromethane solution for subsequent use .
[0069] (2) Preparation of Cefmetazolamide Salt
[0070] a. Stir and cool down 172g of anisole to 0°C, slowly add anisole solution made of 68.8g of aluminum trichloride u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com