Vascular occlusive agent, application thereof and preparation method
A technique for vascular embolizing agent and contrast agent, which is applied in the field of vascular embolizing agent to achieve the effects of high recovery rate, good biodegradability and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
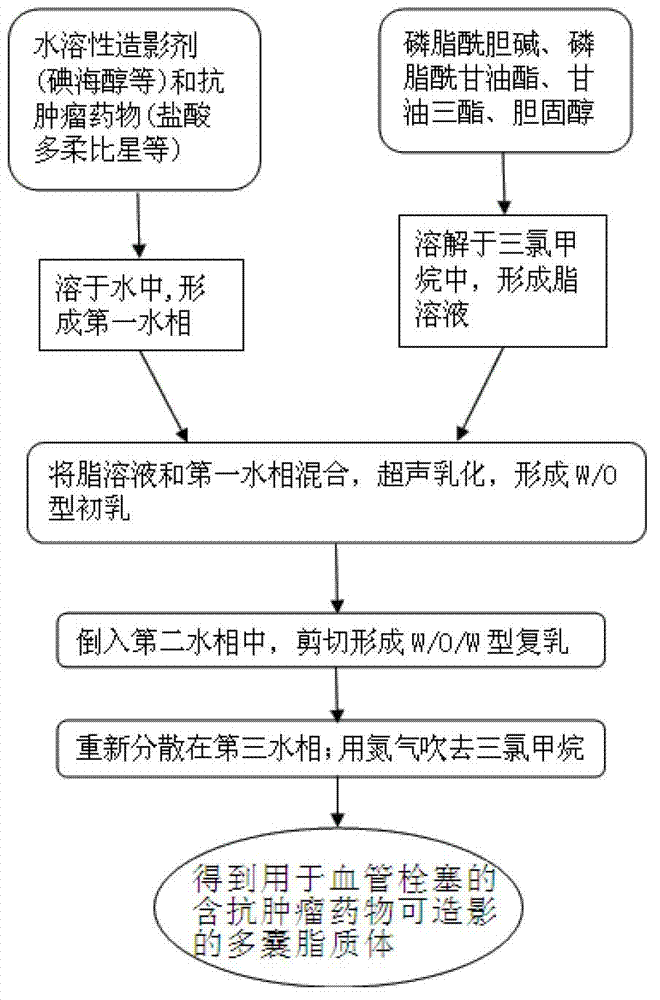
[0043] The preparation of multivesicular liposomes is as follows:
[0044] (1) Weigh 41 mg of phosphatidylcholine dimyristate (phase transition temperature: 23°C), 40 mg of cholesterol, 4.86 mg of phosphatidylglycerol distearate (phase transition temperature: 55°C), three 11.25 mg of glyceryl oleate was dissolved in 1 ml of chloroform to form a lipid solution;
[0045] (2) Mix the lipid solution and the first water phase (1ml ultrapure aqueous solution), and emulsify on an ultrasonic cell crusher for 1-3 minutes to form W / O colostrum; the emulsification temperature is 20°C;
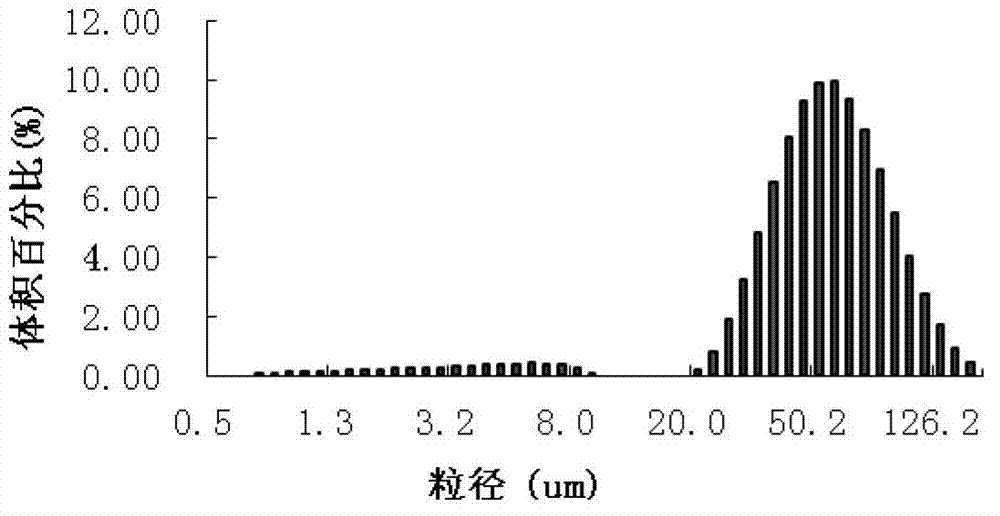
[0046] (3) Pour the W / O type colostrum into the second water phase of equal volume (glucose with a mass percentage concentration of 4% and lysine with a concentration of 20mM, and the volume ratio of the two is 1:1). On the cutter, adjust the shearing speed and time to form a W / O / W type double emulsion with an average particle size of 5-40 μm; the emulsification temperature is 35°C;
[0047] (4) Redispe...
Embodiment 2
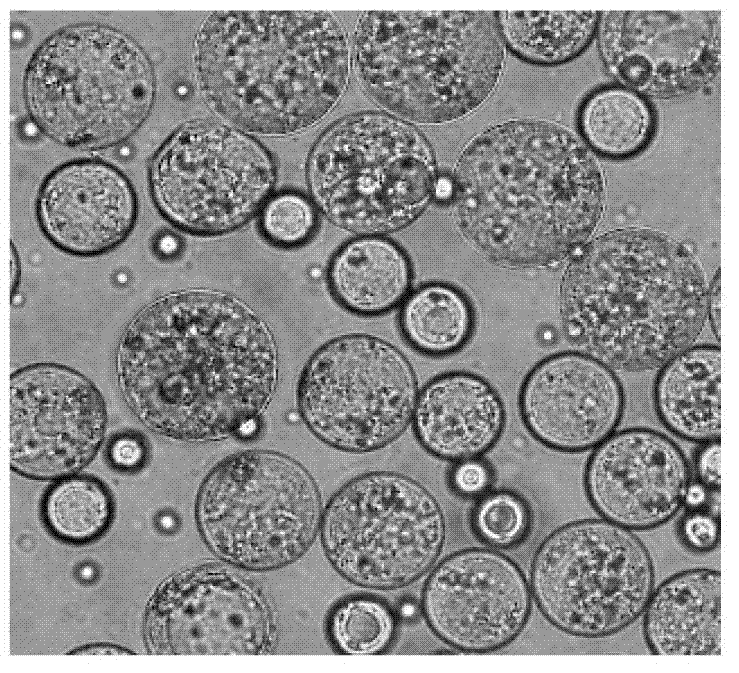
[0050] Preparation of visualized multivesicular liposomes containing antineoplastic drugs, such as figure 1 Shown:
[0051] (1) Weigh 41mg hydrogenated soybean lecithin (phase transition temperature 50°C), 40mg cholesterol, 4.86mg dipalmitate phosphatidylglyceride (phase transition temperature 40°C) and 11.25mg tripalmitin in proportion Dissolve in 1ml of chloroform to form lipid solution;
[0052] (2) 320 mg of contrast agent iohexol and 1 mg of antineoplastic drug doxorubicin were dissolved in 1 ml of ultrapure water to form the first aqueous phase (aqueous drug solution);
[0053] (3) Mix the lipid solution and the aqueous solution, and emulsify on an ultrasonic cell crusher for 1 to 3 minutes to form W / O colostrum; the emulsification temperature is 37°C;
[0054] (4) Pour the W / O type colostrum into the second water phase of equal volume (glucose with a mass percentage concentration of 4% and lysine with a mass percentage of 20mM, the volume ratio of the two is 1:1), and...
Embodiment 3
[0059] Preparation of visualized multivesicular liposomes containing antineoplastic drugs, such as figure 1 Shown:
[0060] (1) Weigh 35.23mg dipalmitate phosphatidylcholine (phase transition temperature: 40°C), 29.1mg cholesterol, and 7.9mg dipalmitate phosphatidylglyceride (phase transition temperature: 23°C) in proportion 30.96 mg of glyceryl trioleate was dissolved in 1 ml of chloroform to form a lipid solution;
[0061] (2) Dissolve 320mg of iodofluhydrin as a contrast agent and 100mg of gemcitabine as an antineoplastic drug in 1ml of ultrapure water to form the first aqueous phase (aqueous drug solution);
[0062] (3) Mix the lipid solution and the aqueous solution, and emulsify on an ultrasonic cell crusher for 1 to 3 minutes to form W / O colostrum; the emulsification temperature is 37°C;
[0063] (4) Pour the W / O colostrum into an equal volume of the second aqueous phase (10% glucose by mass and 50mM lysine, the volume ratio of the two is 1:2). On the high-speed shea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com