Solid phase-hydrothermal preparation method for lithium vanadium phosphate
A lithium vanadium phosphate and hydrothermal technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of low product yield, uneven distribution, cumbersome operation process, etc., and achieve high rate performance, excellent reaction The effect of mild conditions and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
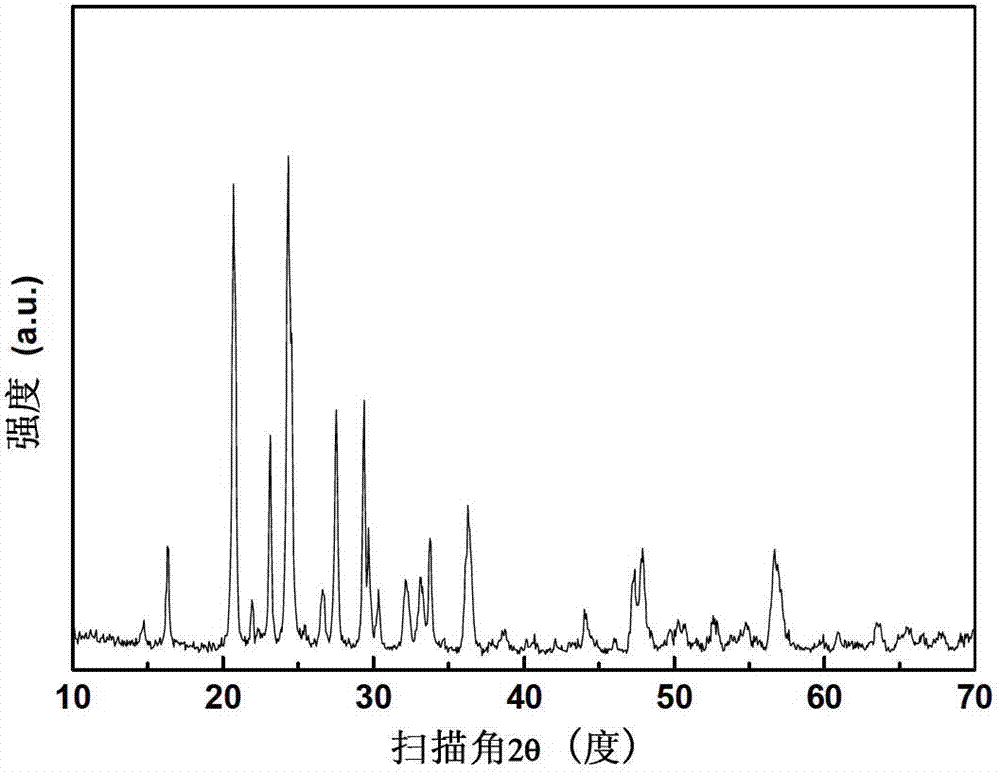
Embodiment 1
[0042] In this example, Li 3 V 2 (PO 4 ) 3 The processing steps of the / C preparation method are as follows:
[0043] (1) Preparation of VPO 4 / C intermediate
[0044] The raw materials and their supplies are: 0.1 mol of vanadium (11.697 g of ammonium metavanadate), 0.1 mol of phosphate (11.503 g of ammonium dihydrogen phosphate), 0.1 mol of carbon (2.8524 g of sucrose);
[0045] Put the supply of the above-mentioned raw materials into the ball mill tank, and add 5mL of distilled water to ball mill to disperse for 0.5h. Heating under protection to 650°C for 10 hours, after the holding time is over, naturally cool to room temperature with the furnace to obtain VPO 4 / C intermediate;
[0046] (2) Hydrothermal preparation of lithium vanadium phosphate
[0047] The raw materials and their supplies are: Lithium 0.09mol (LiOH·H 2 O 3.7764g), phosphate radical 0.03mol (mass fraction 85% phosphoric acid 3.4588g), VPO 4 / C intermediate 0.06mol (8.9305g, carbon content 1.95%);...
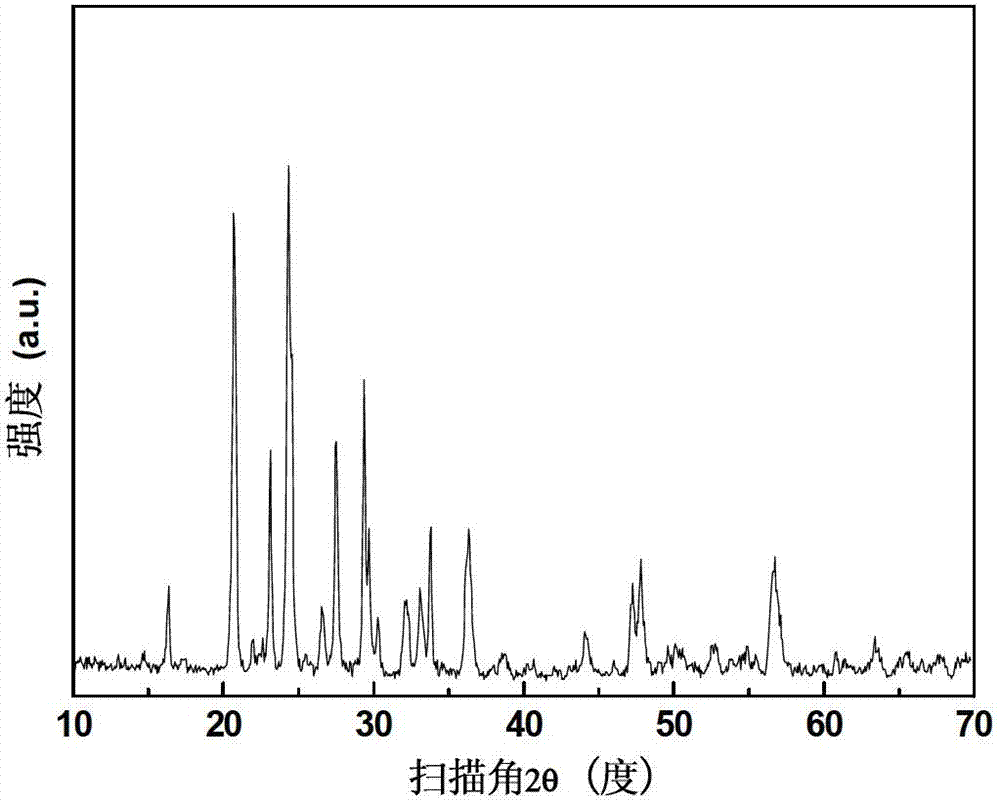
Embodiment 2
[0054] In this example, lithium vanadium phosphate Li 3 V 2 (PO 4 ) 3 The processing steps of the / C preparation method are as follows:
[0055] (1) Preparation of VPO 4 / C intermediate
[0056] The raw materials and their supplies are: 0.1mol of vanadium (9.904g of vanadium pentoxide), 0.1mol of phosphate (13.206g of diammonium hydrogen phosphate), and 0.5mol of carbon (17.5117g of citric acid);
[0057] Put the supply of the above raw materials into the ball mill tank, and add 20mL of acetone to ball mill to disperse for 3h. Heating under protection to 900°C for 4 hours, after the holding time is over, naturally cool to room temperature with the furnace to obtain VPO 4 / C intermediate;
[0058] (2) Hydrothermal preparation of lithium vanadium phosphate
[0059] The raw materials and their supplies are: Lithium 0.09mol (LiOH·H 2 O 3.7764g), phosphate radical 0.03mol (ammonium dihydrogen phosphate 3.4509g), VPO 4 / C intermediate 0.06mol (9.4171g, carbon content 7.55%...
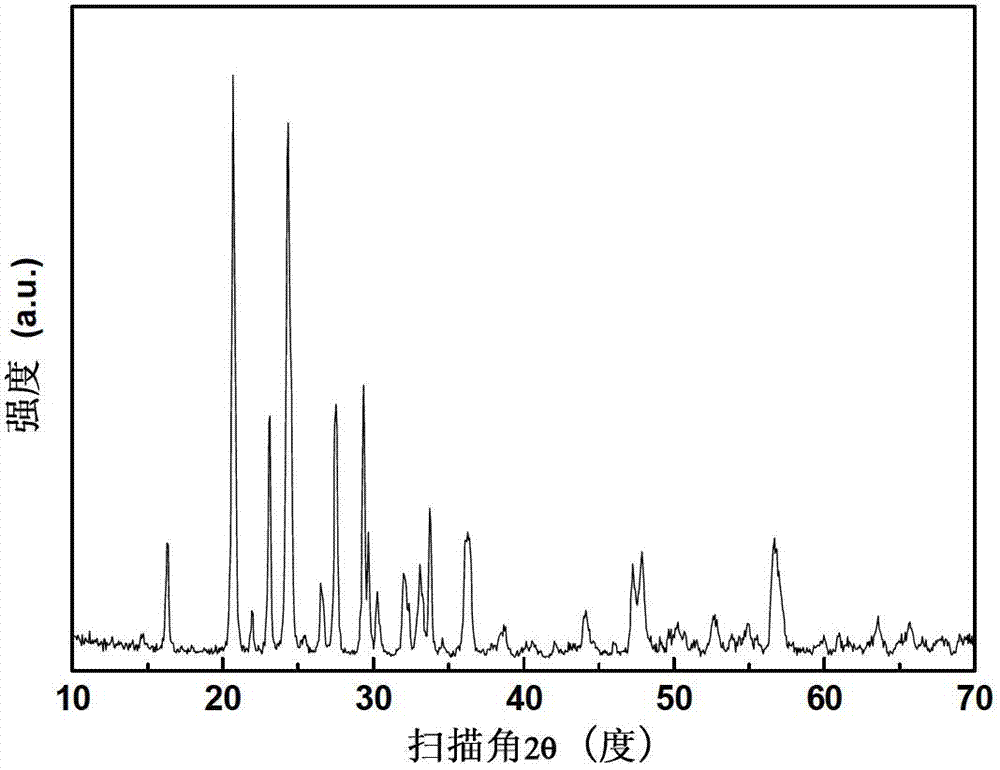
Embodiment 3
[0066] In this example, lithium vanadium phosphate Li 3 V 2 (PO 4 ) 3 The processing steps of the / C preparation method are as follows:
[0067] (1) Preparation of VPO 4 / C intermediate
[0068] The raw materials and their supplies are: 0.1mol of vanadium (11.697g of ammonium metavanadate), 0.1mol of phosphate (13.206g of diammonium hydrogen phosphate), and 0.2mol of carbon (3.16g of stearic acid);
[0069] Put the supply of the above-mentioned raw materials into the ball mill tank, and add 40mL of absolute ethanol to ball mill to disperse for 6h. Heated to 800°C for 6 hours under the protection of nitrogen, and after the holding time was over, naturally cooled to room temperature with the furnace to obtain VPO 4 / C intermediate;
[0070] (2) Hydrothermal preparation of lithium vanadium phosphate
[0071] The raw materials and their supplies are: lithium 0.09mol (lithium acetate 9.1818g), phosphate radical 0.03mol (mass fraction 85% phosphoric acid 3.4588g), VPO 4 / C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com