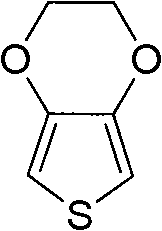
Method for preparing polymer monomer 3,4-ethylenedioxythiophene
A technology of ethylenedioxythiophene and polymer materials, applied in organic chemistry and other directions, can solve problems such as being unsuitable for industrial production, unfavorable cost control, unsuitable for process amplification, etc., to achieve large-scale production, low synthesis cost, Simple and convenient post-processing methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
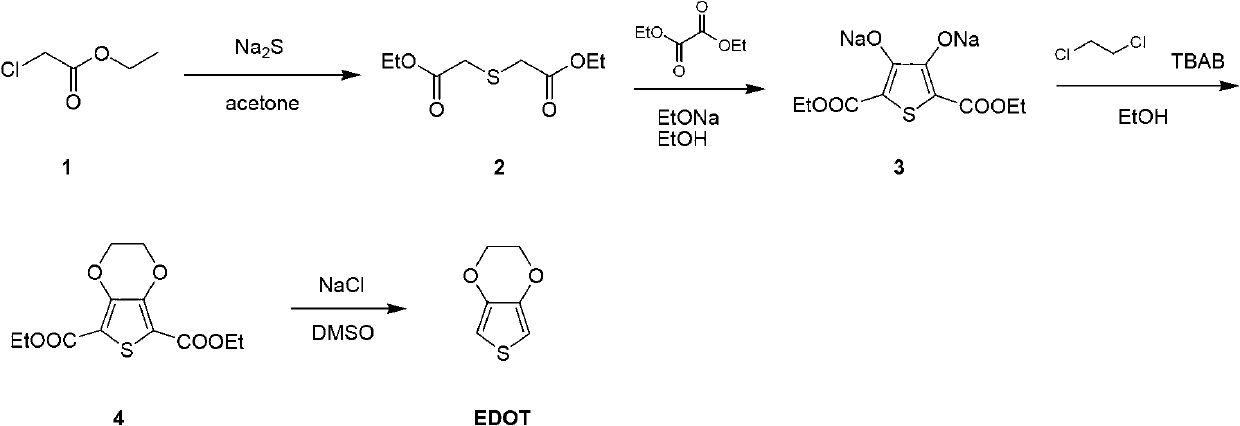
[0045] (1) Synthesis of diethyl thiodiacetate
[0046] Ethyl chloroacetate (245.2g, 2.0mol) was added dropwise to sodium sulfide (78g, 1.0mol) in acetone solution (2L), and stirred at room temperature for 2 hours to generate diethyl thiodiacetate; after monitoring the reaction, Direct filtration, evaporation of solvent to obtain 323 grams of crude product, directly used in the next step without further purification;
[0047] (2) Synthesis of sodium 2,5-dicarboxylate-3,4-thiophenediol
[0048] The raw material (323g) of the previous step was dissolved in ethanol (2L), sodium ethoxide (136g, 2mol) was added in batches at room temperature, diethyl oxalate (146g, 1mol) was slowly added, and the temperature was raised to reflux for 4 hours. After monitoring the completion of the reaction, we obtained The ethanol solution of the intermediate 2,5-dicarboxylate-3,4-thiophenediol sodium, which can be directly used in the next reaction without any purification;
[0049] (3) Synthesis ...
Embodiment 2
[0054] (1) Synthesis of diethyl thiodiacetate
[0055] Ethyl chloroacetate (245.2g, 2.0mol) was added dropwise to sodium sulfide (78g, 1.0mol) in acetone solution (2L), and stirred at room temperature for 4 hours to generate diethyl thiodiacetate; after monitoring the reaction, Direct filtration, evaporation of solvent to obtain 324 grams of crude product, directly used in the next step without further purification;
[0056] (2) Synthesis of sodium 2,5-dicarboxylate-3,4-thiophenediol
[0057] The raw material (324g) of the previous step was dissolved in ethanol (2L), sodium ethoxide (150g, 2.2mol) was added in batches at room temperature, diethyl oxalate (146g, 1mol) was slowly added, and the temperature was raised to reflux for 4 hours. After the reaction was completed, Obtain an ethanol solution of the intermediate sodium 2,5-dicarboxylate-3,4-thiophenediol, which can be directly used in the next reaction without any purification;
[0058] (3) Synthesis of ethyl 3,4-ethyle...
Embodiment 3
[0063] (1) Synthesis of diethyl thiodiacetate
[0064] Ethyl chloroacetate (245.2g, 2.0mol) was added dropwise to sodium sulfide (78g, 1.0mol) in acetone solution (2L), and stirred at room temperature for 6 hours to generate diethyl thiodiacetate; after monitoring the reaction, Direct filtration, evaporation of solvent to obtain 324 grams of crude product, directly used in the next step without further purification;
[0065] (2) Synthesis of sodium 2,5-dicarboxylate-3,4-thiophenediol
[0066] The raw material (324g) of the previous step was dissolved in ethanol (2L), sodium ethoxide (150g, 2.2mol) was added in batches at room temperature, diethyl oxalate (146g, 1mol) was slowly added, and the temperature was raised to reflux for 4 hours. After the reaction was completed, Obtain an ethanol solution of the intermediate sodium 2,5-dicarboxylate-3,4-thiophenediol, which can be directly used in the next reaction without any purification;
[0067] (3) Synthesis of ethyl 3,4-ethyle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com