Catalyst for preparing formate from CO2 and preparation method thereof
A catalyst and formate technology, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of expensive formic acid raw materials, difficult to recycle sulfuric acid, and serious pollution, and achieve high formate The effect of synthetic activity and thermal stability, good reproducibility, and simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Take 5g of organic silicon waste contacts, wash twice with 100mL of ether and ethanol, filter and dry in a vacuum oven at 100°C for 2 hours, put it in a 500mL beaker, add 100mL of dilute hydrochloric acid, at 40°C Heat the reaction for 6h, slowly add sodium carbonate solution, adjust the pH value to 9, heat the reaction at 65°C for 6h, wash, filter, vacuum dry at 100°C for 6h, and calcinate at 350°C for 2h.
[0042] The catalyst material prepared above was subjected to XRD test on the X'Pert PROMPD multifunctional X-ray diffractometer produced by Panalytical Company (Panalytical) in the Netherlands.
[0043] The catalyst material prepared above is carried out ICP test on U.S. Pekin-Elmer Inductively Coupled Plasma Atomic Emission Spectrometer, copper element content is 3.0% (weight ratio, the same below), zinc element content is 0.1% (weight ratio, the same below) .
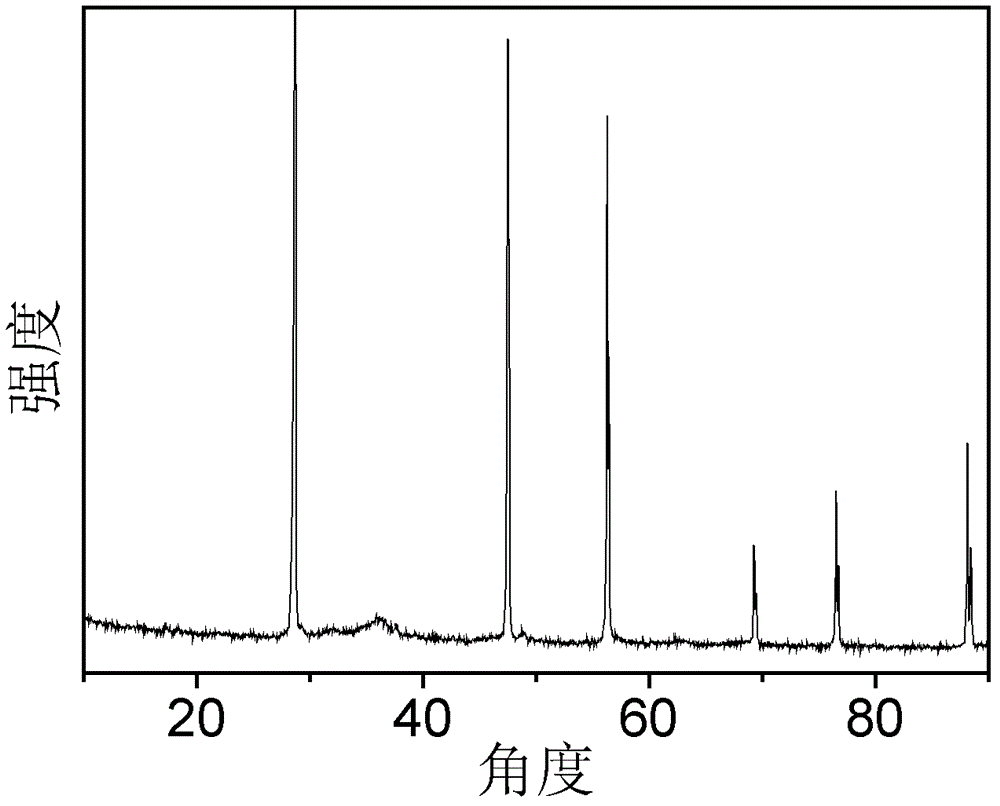
[0044] figure 1 For the XRD pattern of the catalyst obtained in Example 1, wherein 2θ=28.6° is the ch...
Embodiment 2
[0046] Take 5g of organic silicon waste contacts, wash them twice with 100mL of toluene and tetrahydrofuran respectively, filter and dry in a vacuum oven at 100°C for 2 hours, cool to room temperature, put it in a 500mL beaker, add 2gZn(N0 3 ) 2 , add 100mL dilute nitric acid, heat reaction at 60°C for 4h, slowly add ammonia solution, adjust the pH value to 11, heat reaction at 70°C for 4h, wash, filter, vacuum dry at 100°C for 6h, and calcinate at 100°C for 10h. .ICP test results show that the copper element content is 3.3%, and the zinc element content is 4.3%.
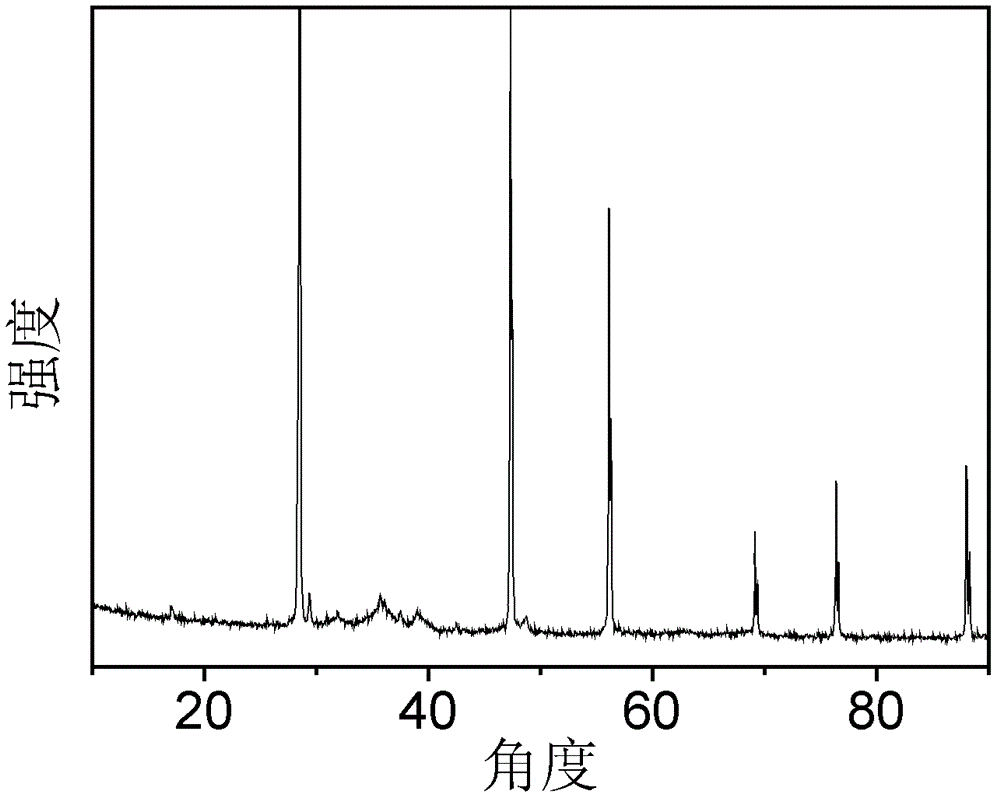
[0047] figure 2 For the XRD pattern of the catalyst obtained in Example 2, wherein 2θ=28.6° is the characteristic peak of Si, the shoulder peak composed of 2θ=35.5° and 2θ=38.7° is the characteristic peak of CuO, and 2θ=36.3° is the characteristic peak of ZnO Peak, it can be seen that the catalyst obtained by this method is a silicon / copper oxide / zinc oxide catalyst.
Embodiment 3
[0049] Take 5g of silicone waste contacts, wash them twice with 100mL of chloroform and acetone respectively, filter and dry in a vacuum oven at 100°C for 2 hours, put them in a muffle furnace, bake at 600°C for 2h, and cool to room temperature . Put the above materials in a 500mL beaker, add 1gZn(NO 3 ) 2 , add 100mL dilute sulfuric acid, heat reaction at 80°C for 2h, slowly add sodium hydroxide solution, adjust pH to 10, heat reaction at 80°C for 2h, wash, filter, vacuum dry at 100°C, and calcinate at 300°C for 5h. ICP test results show that the copper element content is 2.3%, and the zinc element content is 3.9%.
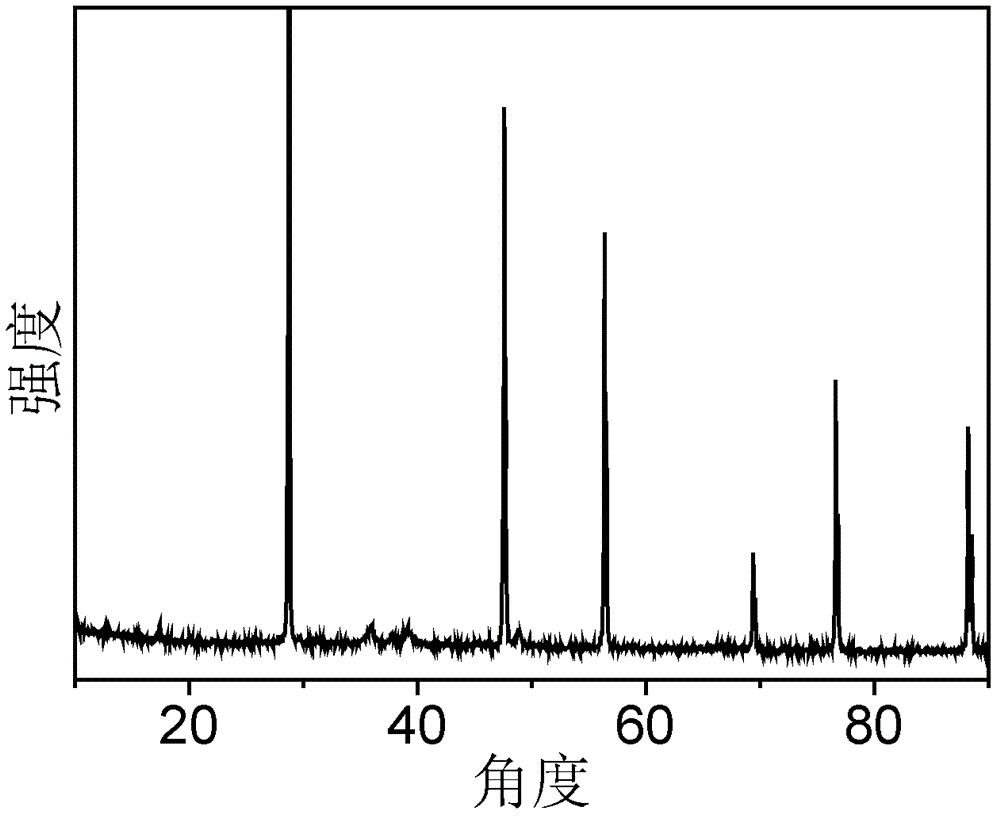
[0050] image 3 For the XRD pattern of the catalyst obtained in Example 3, wherein 2θ=28.6° is the characteristic peak of Si, the shoulder peak composed of 2θ=35.5° and 2θ=38.7° is the characteristic peak of CuO, and 2θ=36.3° is the characteristic peak of ZnO Peak, 2θ=26.6 ° is the characteristic peak of C, thus it can be seen that the catalyst obtained by th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com