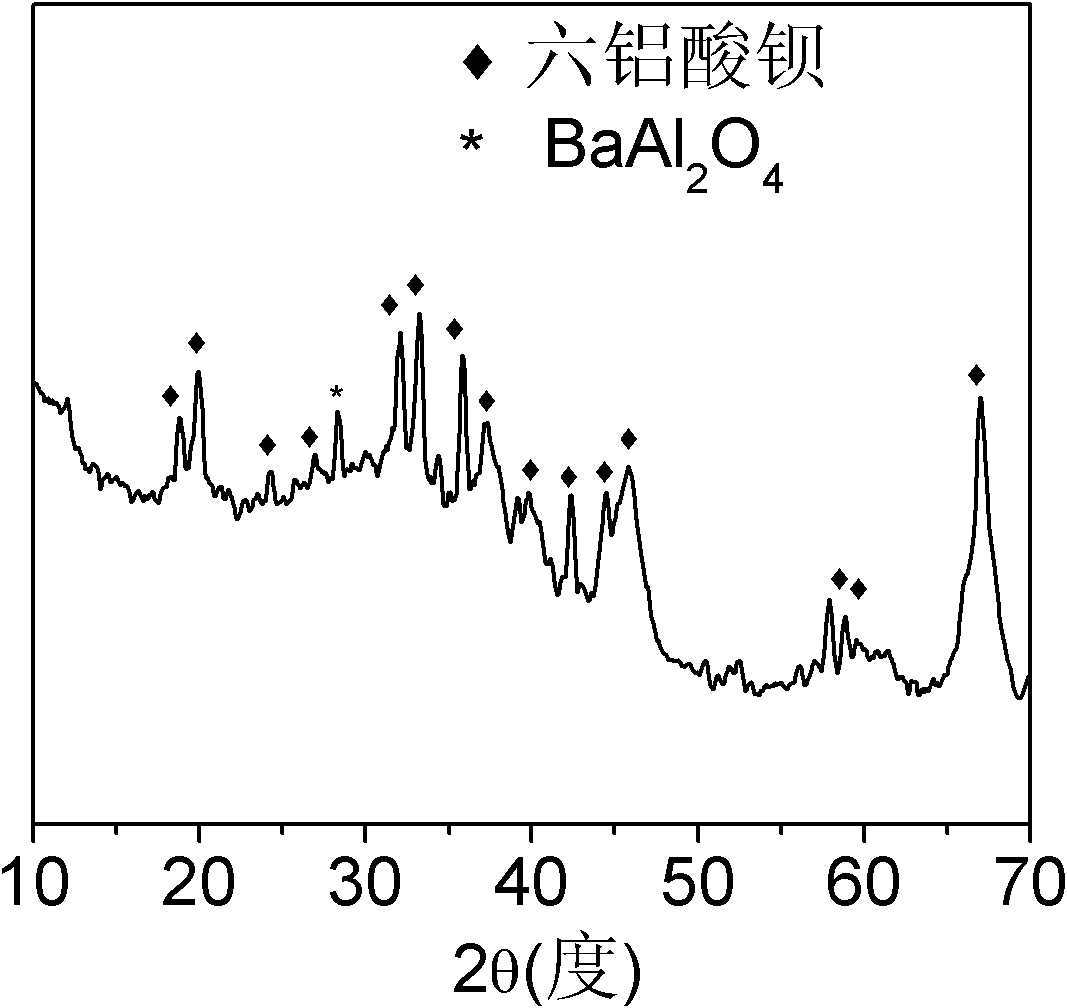
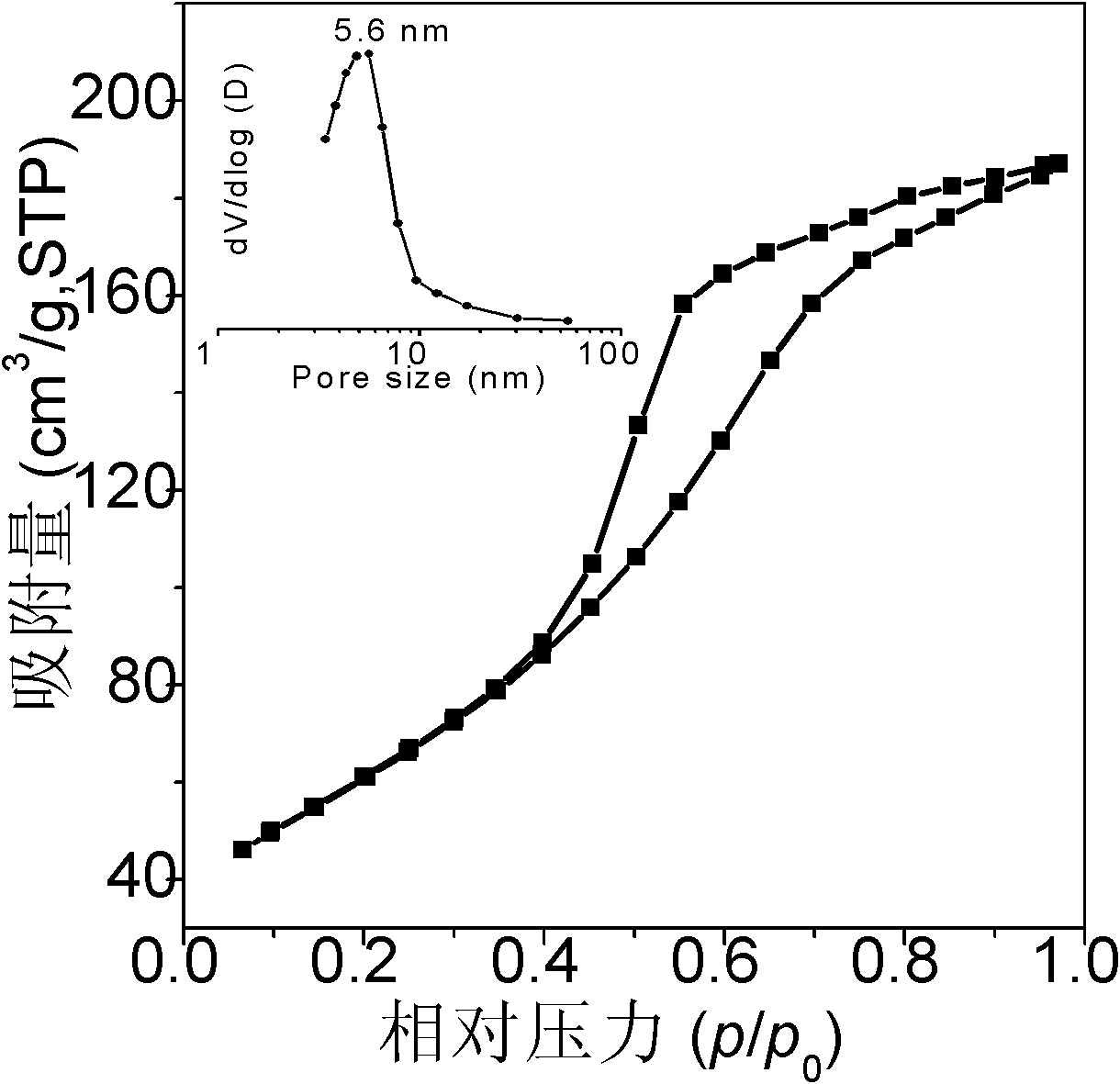
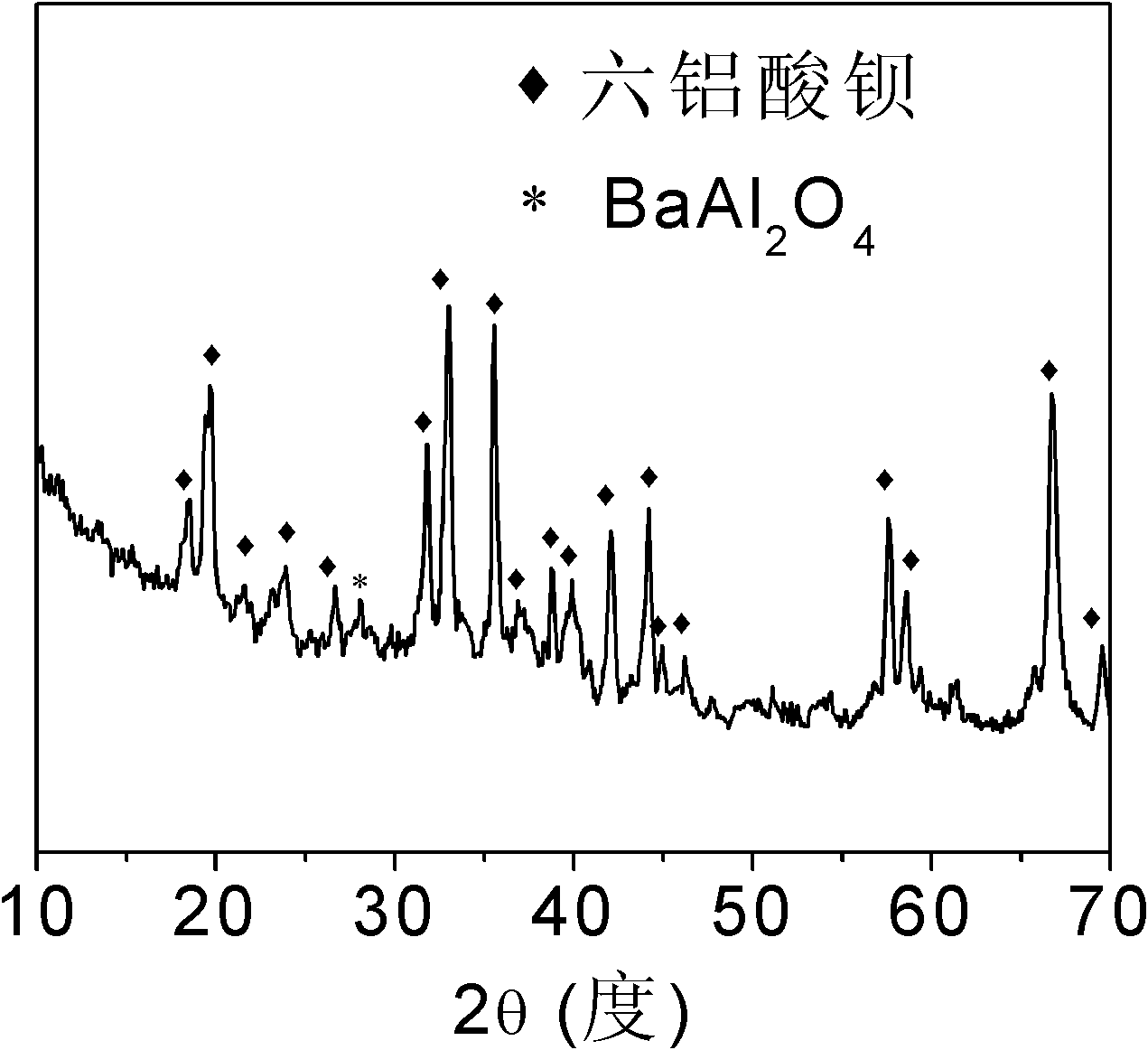
Mesoporous hexaaluminate prepared by carbon template method and having large specific surface area and preparation method of hexaaluminate
A technology of high specific surface area and hexaaluminate, which is applied in the field of mesoporous high specific surface area hexaaluminate and its preparation, can solve the problem that it is difficult to form organic-inorganic mesoporous phase and the uniformity of hexaaluminate particles is not good enough , Inorganic network excessive growth and polymerization and other problems, to achieve the effect of good application prospects, cheap synthetic raw materials, and easy large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of mesoporous barium hexaaluminate: the mesoporous carbon prepared by 1.0g (the mesoporous carbon is prepared by the method in the document J.Am.Chem.Soc.2006,128,11652., the thickness of the pore wall of the obtained mesoporous carbon 6.0 nm, mesopore size 6.2 nm) immersed in 15 mL of nitric acid with a concentration of 5.0 mol / L, wherein the mass ratio of carbon material to solvent was 1:15, treated at 40 ° C for 3 h, filtered, washed and dried to obtain functionalized media porous carbon, and then highly dispersed it in 10mL of ethanol to obtain suspension A; weigh 4.5g of aluminum nitrate, 0.26g of barium nitrate and dissolve them in a mixture of 20mL of water and ethanol (the water / ethanol volume ratio is 1:3 ), the mass ratio of the salt precursor to the solvent is 1: 4.2, and the salt precursor solution B is obtained; then the solution B is added to A, and the resulting mixture is stirred at 20°C and evaporated to dryness to obtain the salt / charcoal C...
Embodiment 2
[0048] Preparation of mesoporous barium hexaaluminate: immerse 1.0 g of prepared mesoporous carbon (pore wall thickness 9.4 nm, mesopore size 7.0 nm) in 10 mL of hypochlorous acid solution with a concentration of 2.0 mol / L, wherein the carbon material and The mass ratio of the solvent is 1:10, treated at 40°C for 8 hours, filtered, washed and dried to obtain functionalized mesoporous carbon, and then highly dispersed in 10mL of ethanol to obtain suspension A; weigh 4.5g of aluminum nitrate , 0.25g barium acetate is dissolved in the mixed solution of 38mL water and acetone (water / acetone volume ratio is 3: 1), the mass ratio of salt precursor and solvent is 1: 8, makes salt precursor solution B; Then Add solution B to A, stir the resulting mixture at 40°C, and evaporate to dryness to obtain a salt / charcoal compound; put the salt / charcoal compound into a sand core funnel, place it in a 20mL container with a concentration of 6.0mol / In a hydrothermal kettle with L ammonium carbon...
Embodiment 3
[0053] Preparation of mesoporous barium hexaaluminate: Immerse 1.0 g of prepared mesoporous carbon (pore wall thickness 8.0 nm, mesopore size 6.5 nm) in 5 mL of 8.0 mol / L sulfuric acid, wherein the mass ratio of carbon material to solvent is 1:5, treated at 90°C for 5 hours, filtered, washed and dried to obtain functionalized mesoporous carbon, and then highly dispersed in 10mL ethanol to obtain suspension A; weigh 13.5g aluminum nitrate, 0.75g barium acetate Dissolve in the mixed solution of 28.5mL water and propanol (water / propanol volume ratio is 1: 1), the mass ratio of salt precursor and solvent is 1: 2, makes salt precursor solution B; Then solution B was added to A, and the resulting mixture was stirred at 50°C and evaporated to dryness to obtain a salt / charcoal composite; the salt / carbon composite was put into a funnel with a sand core, placed in a 20mL urea with a concentration of 2.0mol / L In the hydrothermal kettle of the aqueous solution, after standing at 110°C for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com