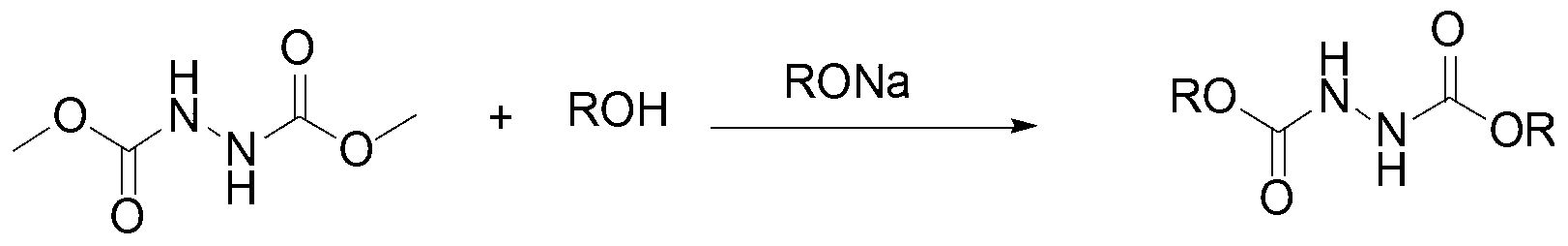
Esterification method for preparing hydrogenated dialkyl azodicarbonic acid
A technology for dialkyl hydroazodicarboxylate and dimethyl hydroazodicarboxylate, which is applied in the field of dialkyl hydroazodicarboxylate represented by the formula ROOCNHHNCOOR, can solve the problem of non-compliance, environment and operators Harm, production equipment corrosion and other problems, to achieve the effect of low production cost, short process route, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 dipropyl hydroazodicarboxylate
[0028] In a 250mL three-necked round-bottomed flask equipped with a mechanical stirrer, a condensing reflux device and a thermometer, add 100mL of n-propanol, and under nitrogen protection, add 0.14g (0.006mol) of sodium metal. After the sodium metal is completely dissolved, add dropwise 14.80 g of dimethyl hydroazodicarboxylate was reacted for 18 hours under reflux in an oil bath, and the reaction was stopped. The pH of the reaction solution was adjusted to 6 with 10% hydrochloric acid (mass fraction, the same below), and the organic phase was extracted three times with dichloromethane, combined and dried with anhydrous sodium sulfate overnight (10h), and the unreacted normal Propanol was recovered, and recrystallized with ethyl acetate-petroleum ether mixture (the volume ratio of the two was 1:8) to obtain 17.41 g of dipropyl hydroazodicarboxylate as a white solid, with a yield of 85.3%.
[0029] 1 HNM...
Embodiment 2
[0032] The preparation of embodiment 2 diisobutyl hydroazodicarboxylate
[0033] In a 250mL three-necked round-bottomed flask equipped with a mechanical stirrer, a condensing reflux device and a thermometer, add 100mL of isobutanol, and under nitrogen protection, add 0.14g (0.006mol) of sodium metal. After the sodium metal is completely dissolved, add dropwise 14.80 g of dimethyl hydroazodicarboxylate was reacted for 16 hours under reflux in an oil bath, and the reaction was stopped. The pH value of the reaction solution was adjusted to 5 with 10% hydrochloric acid solution, the organic phase was extracted three times with dichloromethane, the organic phase was combined, and dried with anhydrous sodium sulfate overnight (10h), the unreacted isobutanol was evaporated to recover, and the acetic acid Ethyl ester-petroleum ether mixture (the volume ratio of the two is 1:12) was recrystallized to obtain 20.60 g of diisobutyl hydroazodicarboxylate as a white solid, with a yield of 8...
Embodiment 3
[0037] The preparation of embodiment 3 dipentyl hydroazodicarboxylates
[0038] In a 250mL three-necked round-bottomed flask equipped with a mechanical stirrer, a condensing reflux device and a thermometer, add 100mL of isoamyl alcohol, and under nitrogen protection, add 0.14g (0.006mol) of sodium metal. After the sodium metal is completely dissolved, add 14.80 g dimethyl hydroazodicarboxylate, reacted for 12 hours under reflux in an oil bath, and stopped the reaction. The pH of the reaction solution was adjusted to 5 with 10% hydrochloric acid solution, the organic phase was extracted three times with dichloromethane, the organic phase was combined, and dried overnight (8h) with anhydrous sodium sulfate, the unreacted isoamyl alcohol was evaporated to recover, and the acetic acid Ethyl ester-petroleum ether mixture (the volume ratio of the two is 1:10) was recrystallized to obtain 23.72 g of dipentyl hydroazodicarboxylate as a white solid, with a yield of 91.2%.
[0039] 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com