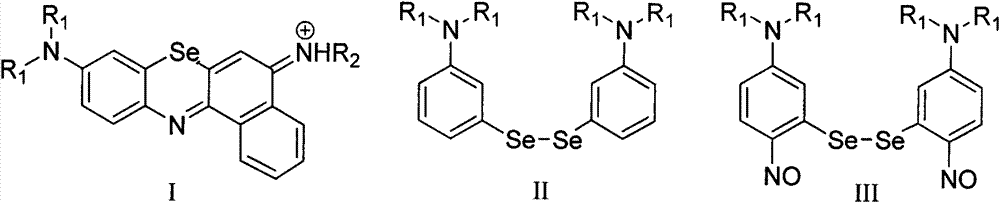
Method for preparing benzo-phenoselenazine photosensitizer
A photosensitizer, benzophene technology, applied in the field of preparation of new photosensitizer benzophene selenazine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of nano-copper oxide
[0035] CuSO 4 +NaOH+NaCO 3 → Cu 2 (OH) 2 CO 3 →CuO+H 2 O+CO 2
[0036] Prepare 1mol·L -1 CuSO 4 Aqueous solution, 1mol L -1 NaOH aqueous solution, 0.5mol L -1 NaCO 3 aqueous solution, add CuSO in a beaker 4 100mL each of aqueous solution and NaOH aqueous solution. At this time, a blue flocculent precipitate is formed, and then Na 2 CO 3 Aqueous solution 260mL, the reaction produces Cu 2 (OH) 2 CO 3Precipitation, vacuum filtration, the obtained precipitation was washed several times with deionized water, until there was no SO in the filtrate with barium chloride solution. 4 2- , the precipitate was dried in an oven at 90°C for 3h, then ground, and calcined in a muffle furnace at 350°C for 4h to obtain black CuO powder.
Embodiment 2
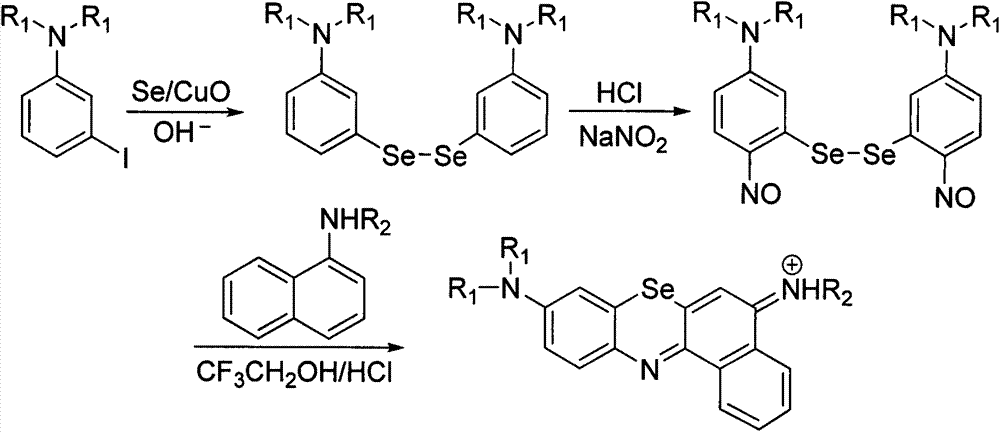
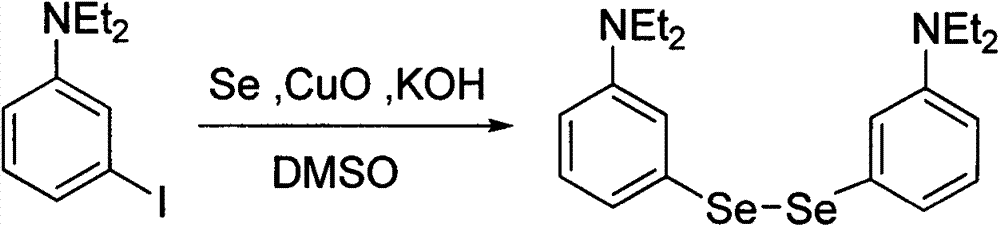
[0037] Embodiment 2: the preparation of two-(3-N, N-diethylaminophenyl) diselenium
[0038]
[0039] In a 100mL round bottom flask, mix 2g (7.2mmol) of 3-iodo-N, N-diethylaniline, 1.2g (15mmol) of copper oxide powder, 1.15g (14.5mmol) of selenium powder, 0.81g (14.5mmol) of potassium hydroxide ) and dimethyl sulfoxide 20mL, the solid was completely dissolved and reacted overnight at 110°C under the protection of argon, cooled to room temperature, adding 30mL of mixed solvent of water and ethyl acetate (volume ratio: 1:1) to the reaction flask, and using Separate the organic phase and the aqueous phase with a 250mL separatory funnel, extract the aqueous phase three times with ethyl acetate, combine the organic phases, wash twice with saturated NaCl solution, and add anhydrous NaCl after the organic phase is clear and free of oily substances. 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 2.36 g of a light red liquid with a yield of 71%. ...
Embodiment 3
[0041] Embodiment 3: Preparation of two-(3-N, N-diethylamino-6-nitrosobenzene) diselenium
[0042]
[0043] Under ice bath, 2.36g (5.2mmol) of the diselenium product obtained in the previous step was dissolved in 1mol L -1 In 200mL of hydrochloric acid, slowly add 0.72g (11.4mmol) of saturated aqueous solution of sodium nitrite, the solution slowly turns from yellow to orange during the dropwise addition, and a precipitate is formed, NaNO 2 After the solution was added dropwise, it was stirred for 10 min to complete the reaction. Filtration, the filtrate was extracted with dichloromethane (3 × 100mL), the organic layers were combined, washed twice with saturated NaCl solution, anhydrous NaCl 2 SO 4 After drying, the solvent was distilled off under reduced pressure, and the obtained yellow-brown crude product was recrystallized from isopropanol to obtain 1.84 g of orange powdery solid with a yield of 70%.
[0044] 1 H NMR (CDCl 3 , 400MHz, ppm) δ8.38(d, J=9.6Hz, 2H), 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com