Phenols electrochemical sensor based on ionic liquid-graphene oxide sensitive membrane
A technology of ionic liquid and graphene, applied in the field of electroanalytical chemistry, can solve the problems of cumbersome operation, time-consuming, lack of high sensitivity and selectivity, etc., and achieve simple operation process, simple instrument requirements, good conductivity and enrichment effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
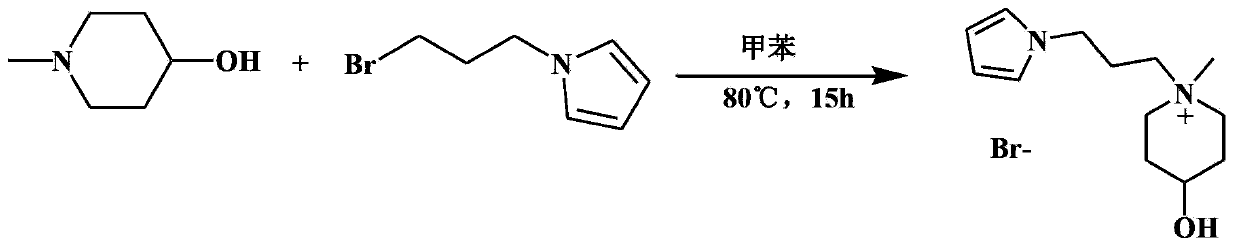
[0038] A novel ionic liquid 4-hydroxyl-1-methyl-1-(3-pyrropropyl)-piperidinium bromide, the synthesis method steps are as follows:
[0039] Weigh 4-hydroxy-N-methylpiperidine (0.4549g, 3mmol) in a 50mL three-necked flask, add 15mL of toluene as solvent, add 1-(3-bromopropyl)pyrrole (0.5640g, 3.3mmol) dropwise Put it into a three-necked flask, after the addition, put it in an oil bath at 80°C, stir and react for 15 hours under the protection of nitrogen, and a yellow oily insoluble liquid is formed. Cool to room temperature, separate the toluene layer, add water to dissolve the yellow oily liquid, and extract with ether. The water layer was evaporated to dryness to obtain a yellow oily liquid, namely the product 4-hydroxyl-1-methyl-1-(3-pyrropropyl)-piperidinium bromide ionic liquid of the present invention 0.8510g (yield 93.9%), 1 H NMR (D 2O) δ: 6.76(d,2H), 6.11(d,2H), 3.98(t,2H), 3.89(d,1H), 3.38(t,2H), 3.20(d,2H), 3.12(d,2H) ), 2.92 (s, 3H), 2.16 (t, 2H), 1.96 (d, 2H), 1...
Embodiment 2
[0042] A kind of carboxylated graphene oxide-ionic liquid composite nanomaterial, its preparation method steps are as follows:
[0043] (1) 10mg graphene oxide (GO), 0.5g sodium hydroxide solid (NaOH) and 0.5g chloroacetic acid (ClCH 2 COOH) was dissolved in 10 mL of water, homogenized by ultrasonication, and stirred on a magnetic stirrer for 2 hours. After centrifugation, the solid was washed with water until neutral, and dried in vacuum at 60°C to obtain 9.5 mg of carboxylated graphene oxide, which was designated as GO-COOH. All the obtained carboxylated graphene oxide was added to 10mL0.01mol L -1 In NaOH solution, the reaction was stirred on a magnetic stirrer for 2 h, centrifuged, the solid was washed with water until neutral, and dried in vacuum at 60°C to obtain 9.0 mg of carboxylated graphene oxide in an alkalized form, which was designated as GO-COONa.
[0044] There are no special requirements for the graphene oxide used;
[0045] Specifically, the graphene oxide ...
Embodiment 3
[0051] Such as Figure 4 As shown, a carboxylated graphene oxide-ionic liquid composite nanomaterial modified electrode includes an insulating layer 1, a glassy carbon substrate 3, and an electrode lead 4 electrically connected to the glassy carbon substrate 3. The surface of the glassy carbon substrate 3 is coated with carboxylated Graphene oxide-ionic liquid nanocomposite nanomaterial membrane2. The preparation method of the above-mentioned carboxylated graphene oxide-ionic liquid modified electrode comprises the following steps in sequence:
[0052] (1) Glassy carbon electrode pretreatment: firstly polish the glassy carbon electrode (3 mm in diameter) with alumina polishing powder with a particle size of 0.3 micron and 0.05 micron, rinse with water, and then use nitric acid solution (65wt% Nitric acid and water equal volume mixed solution), ethanol solution (95wt% ethanol and water equal volume mixed solution) and water ultrasonically clean the glassy carbon electrode for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com