Post-column derivatization preparation method and application of aminoglycosides compound
A post-column derivatization and compound technology, applied in the field of veterinary drug residue analysis, can solve problems that are difficult to control and difficult to find
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
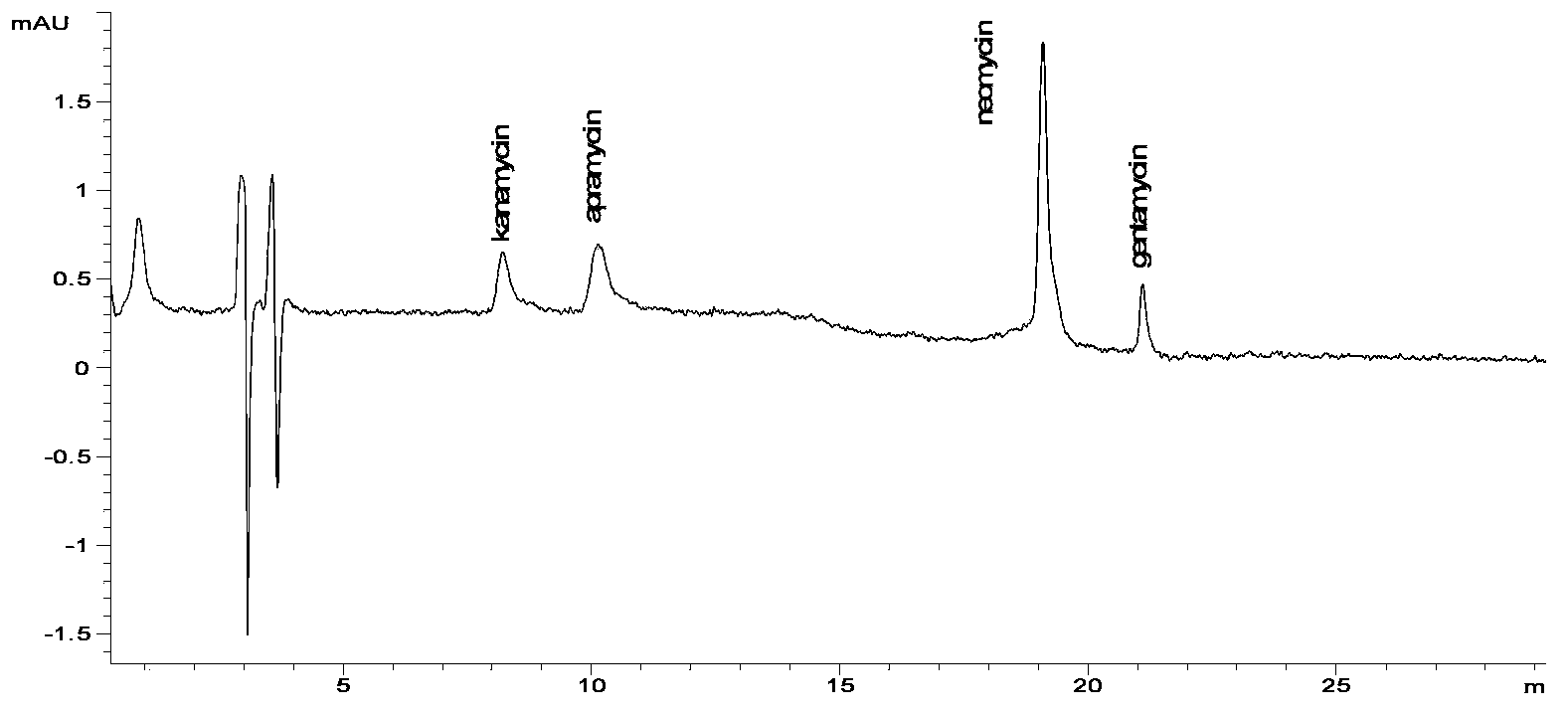
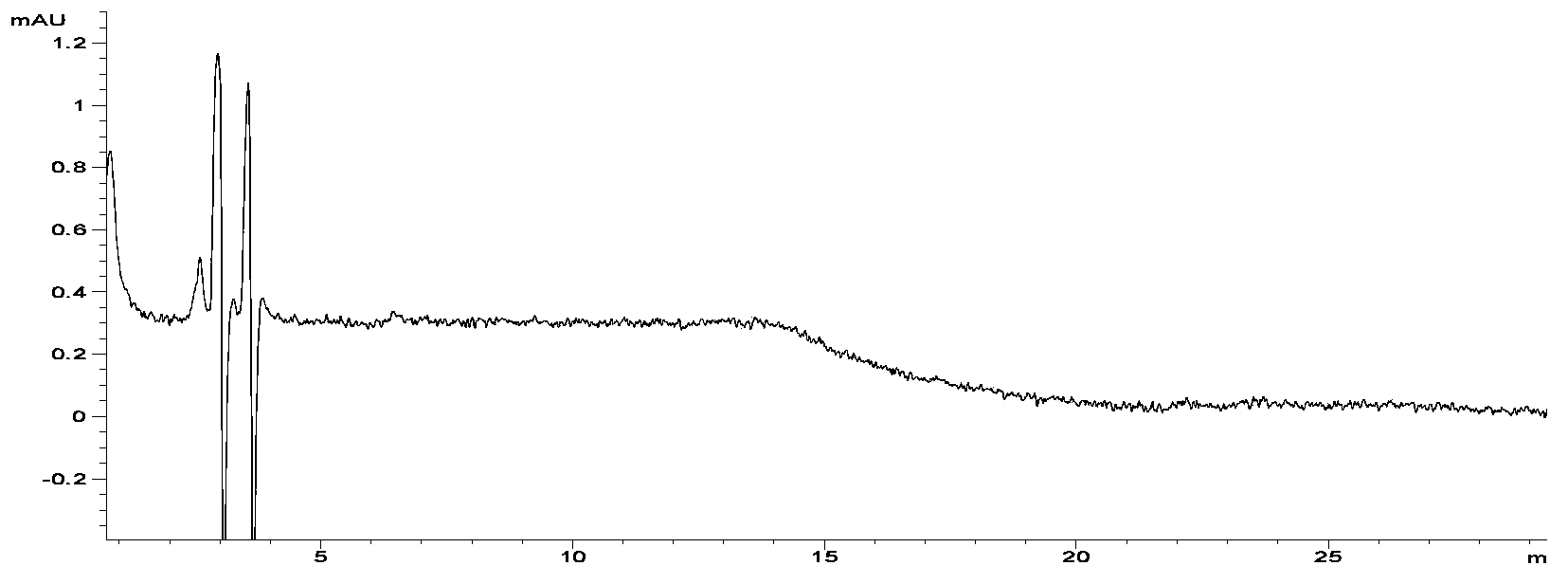
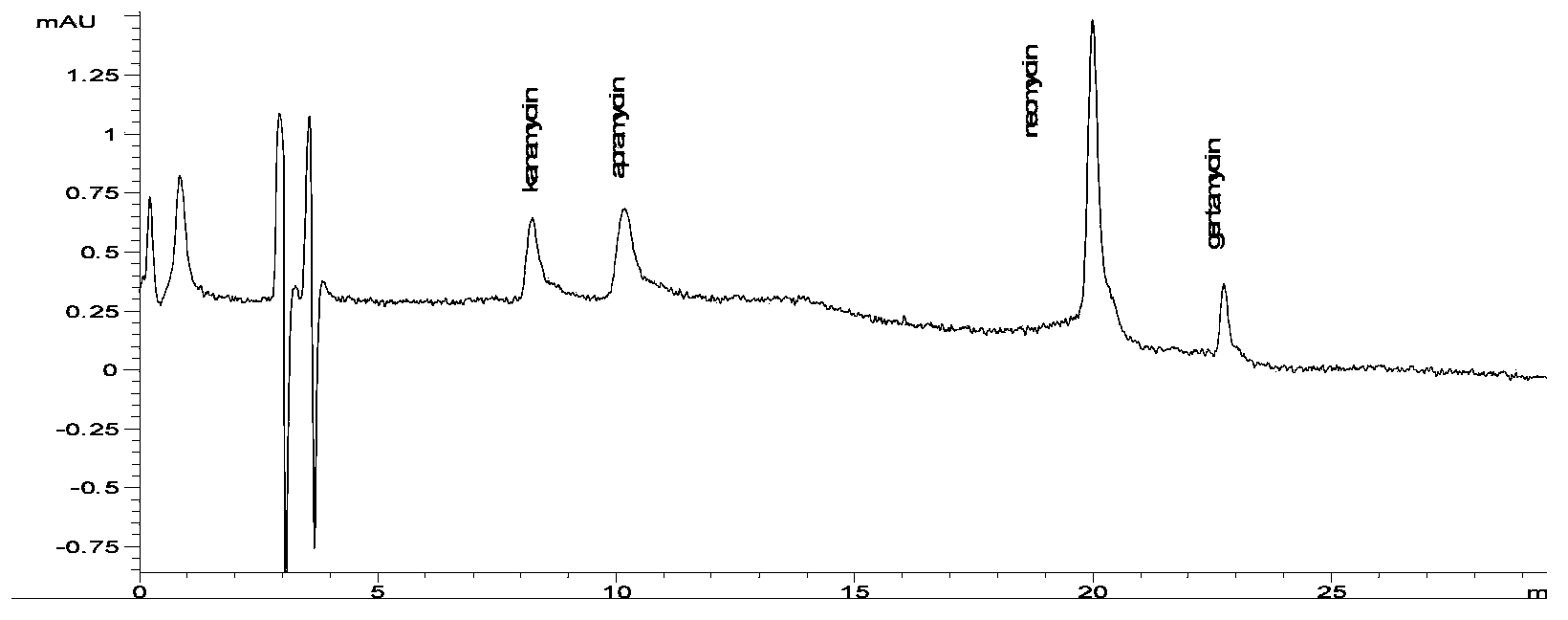
[0013] In this example, a high performance liquid chromatography (HPLC) separation and detection method for standard samples of aminoglycoside compounds was first established, and then the conditions for derivatization reaction with o-phthalaldehyde were established, and finally the samples were analyzed and detected.
[0014] Specific steps are as follows:
[0015] 1. Purification treatment of aminoglycosides
[0016] Extract with phosphate buffer (weigh 8.50g of sodium chloride, 3.58g of disodium hydrogen phosphate, 3.12g of sodium dihydrogen phosphate, add water to dissolve and dilute to 1000mL) and 10% trichloroacetic acid solution, C 18 SPE column purification, eluting with methanol, collecting the eluate, blowing dry with nitrogen in a water bath at 40°C, dissolving in 1.0 mL of water, transferring to a 1.5 mL polypropylene centrifuge tube, centrifuging at 10,000 r / min for 10 min, and taking the supernatant for high performance liquid chromatography analyze.
[0017] 2...
Embodiment 2
[0042] Embodiment 2: the mensuration of sample
[0043] (1) Extraction of samples
[0044] Select pig kidney, pig muscle, bovine kidney and bovine muscle, chicken muscle and milk as test samples, and carry out single or multiple residue detection of apramycin, kanamycin, neomycin and gentamicin. The concentrations of kanamycin, apramycin and gentamicin added to milk were 20μg / L, 50μg / L, 100μg / L and 200μg / L respectively, and the concentration of neomycin was 100μg / L, 500μg / L, 1000μg / L and 2000μg / L; the concentration of gentamicin added in pig kidney, cow kidney, pig muscle, cow muscle and chicken muscle was 50μg / kg, 100μg / L kg and 200μg / kg three added concentrations, set a blank sample respectively, repeat each concentration 5 times, and carry out 5 separate experiments to calculate the recovery rate and variation coefficient with the commonly used external standard method
[0045] The sample is extracted with 10% trichloroacetic acid solution of phosphate buffer (the formul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com