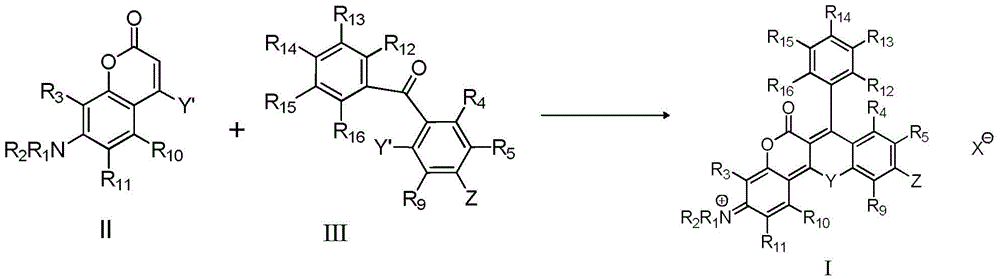
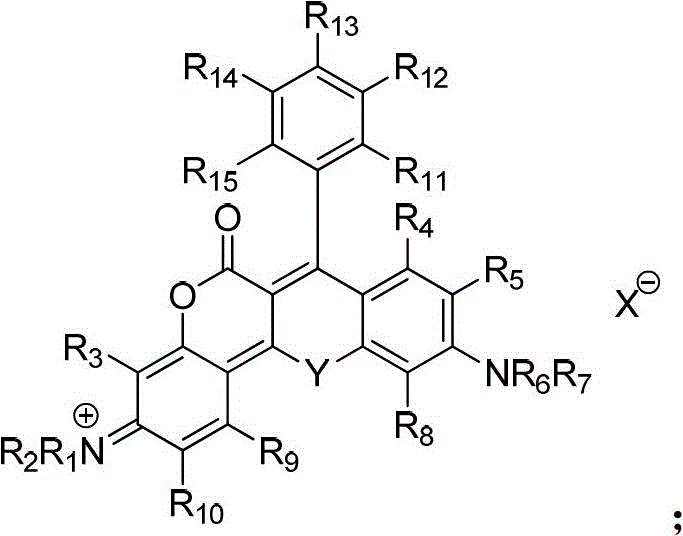
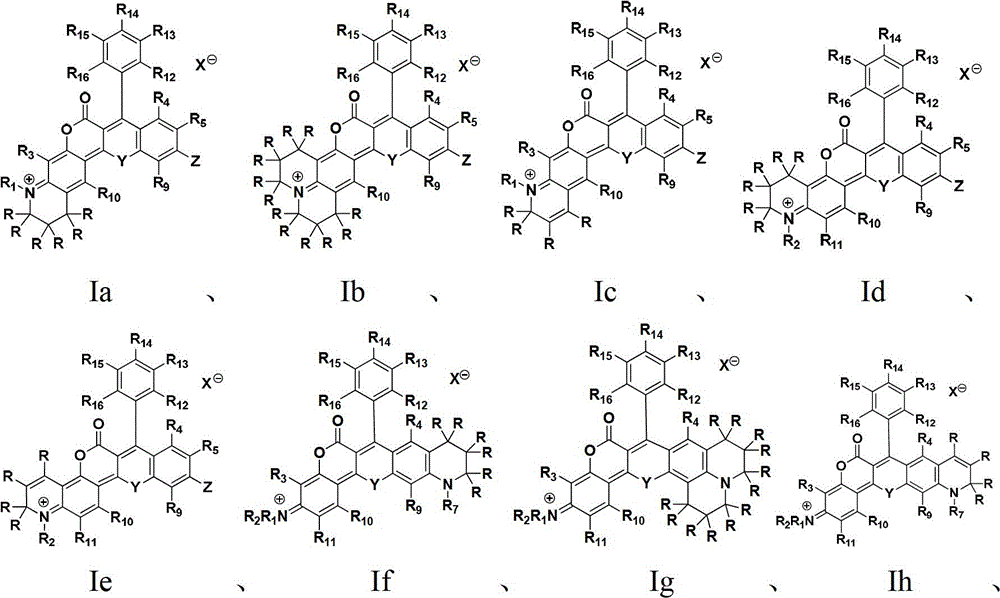
A class of near-infrared fluorescent dyes based on rhodamine and its preparation method and application
A fluorescent dye and near-infrared technology, applied in luminescent materials, fluorescence/phosphorescence, coumarin dyes, etc., can solve the problems of large Stokes shift, application limitations, short absorption and emission wavelengths, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140]
[0141] 7-Dimethylamino-4-hydroxycoumarin II-1 was prepared from 3-N,N-dimethylaminophenol according to literature (Chem. Commun., 2006, 3886-3888), with a total yield of 80%.
[0142] Mix 0.233g (0.001mol) of compound II-1 and 0.313g (0.001mol) of compound III-1 in 10ml of tetrachloroethane, add 1.42g (0.01mol) of phosphorus pentoxide in batches, and reflux for 5 hours , add 20 ml of water after cooling, extract three times with 20 ml of chloroform, after the chloroform phase is dried, evaporate the solvent under reduced pressure, dissolve the resulting product in 10 ml of ethanol, add 2 ml of perchloric acid (70%), slowly drop Distilled water, precipitated solid, filtered, vacuum dried, and purified by column chromatography to obtain 0.22 g of compound I-1 with a yield of 36.1%. ESI MS: m / z, 511.2. lambda ab. max / nm=595nm,λ em max / nm=648nm,Ф f =0.32.
[0143] Dissolve 0.194g (0.33mmol) of compound I-1, 0.038g (0.33mmol) of N-hydroxysuccinimide, and dicycl...
Embodiment 2
[0145]
[0146] Compound II-2 was prepared from 8-hydroxyjuloridine according to the literature (Chem.Commun., 2006, 3886–3888), with a total yield of 75%; Compound III-2 was prepared according to the literature (J.Arden-Jacob, Ph.D .Thesis, Verlag Shaker, Aachen, 1993.) Prepared from 8-hydroxy-juloridine and phthalic anhydride with a yield of 20%.
[0147] Mix 0.257g (0.001mol) of compound II-2 and 0.337g (0.001mol) of compound III-2 in 10ml of tetrachloroethane, add 0.71g (0.005mol) of phosphorus pentoxide in batches, and reflux for 8 hours , add 20 ml of water after cooling, extract three times with 20 ml of chloroform, after the chloroform phase is dried, evaporate the solvent under reduced pressure, dissolve the resulting product in 10 ml of ethanol, add 2 ml of perchloric acid (70%), slowly drop Distilled water, precipitated a solid, filtered, dried in vacuo, and purified by column chromatography to obtain 0.25 g of compound I-3 with a yield of 36.1%. ESI MS: m / z, 55...
Embodiment 3
[0150]
[0151] Compound II-3 was prepared from 7-hydroxy-1-methyl-1,2,3,4-tetrahydroquinoline according to literature (Chem.Commun., 2006, 3886-3888), with a total yield of 85%; Compound III -3 According to the literature (J.Arden-Jacob, Ph.D.Thesis, VerlagShaker, Aachen, 1993.) from 7-hydroxy-1-methyl-1,2,3,4-tetrahydroquinoline and phthalate Preparation of formic anhydride with a yield of 25%.
[0152] Mix 0.231g (0.001mol) of compound II-3 and 0.311g (0.001mol) of compound III-3 in 10ml of tetrachloroethane, add 1.42g (0.01mol) of phosphorus pentoxide in batches, and reflux for 2 hours , add 20 ml of water after cooling, extract three times with 20 ml of chloroform, after the chloroform phase is dried, evaporate the solvent under reduced pressure, dissolve the resulting product in 10 ml of ethanol, add 2 ml of perchloric acid (70%), slowly drop Distilled water, precipitated solid, filtered, vacuum dried, and purified by column chromatography to obtain 0.21 g of compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com